Trung Tâm Luyện Thi Đại Học
Chapter 2. Acids Bases and Salt
2.2. What Do All Acids And All Bases Have In Common?
In Section 2.1 we have seen that all acids have similar chemical properties. What leads to this similarity in properties? We saw in Activity 2.3 that all acids generate hydrogen gas on reacting with metals, so hydrogen seems to be common to all acids. Let us perform an Activity to investigate whether all compounds containing hydrogen are acidic.
Activity 2.8
- Take solutions of glucose, alcohol, hydrochloric acid, sulphuric acid, etc.
- Fix two nails on a cork, and place the cork in a 100 mL beaker.
- Connect the nails to the two terminals of a 6 volt battery through a bulb and a switch, as shown in Fig. 2.3.
- Now pour some dilute HCl in the beaker and switch on the current.
- Repeat with dilute sulphuric acid.
- What do you observe?
- Repeat the experiment separately with glucose and alcohol solutions. What do you observe now?
- Does the bulb glow in all cases?
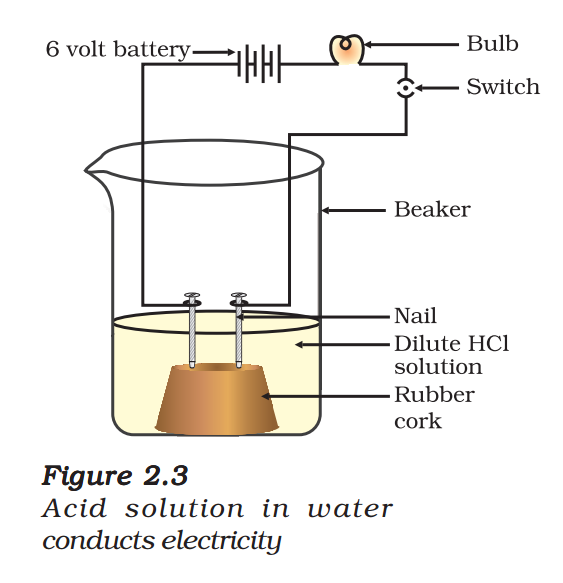
The bulb will start glowing in the case of acids, as shown in Fig. 2.3. But you will observe that glucose and alcohol solutions do not conduct electricity. Glowing of the bulb indicates that there is a flow of electric
current through the solution. The electric current is carried through the acidic solution by ions.
Acids contain H+ ion as cation and anion such as Cl– in HCl, \( NO_{3}^{-} \) in \( HN{{O}_{3}},\,\,SO_{4}^{2-} \) in \( {{H}_{2}}S{{O}_{4}},\,\,C{{H}_{3}}CO{{O}^{-}} \) in CH3COOH. Since the cation present in acids is H+, this suggests that acids produce hydrogen ions, H+(aq), in solution, which are responsible for their acidic properties.
Repeat the same Activity using alkalis such as sodium hydroxide, calcium hydroxide, etc. What can you conclude from the results of this Activity?
2.2.1 What Happens to an Acid or a Base in a Water Solution?
Do acids produce ions only in aqueous solution? Let us test this.
Activity 2.9
- Take about 1g solid NaCl in a clean and dry test tube and set up the apparatus as shown in Fig. 2.4.
- Add some concentrated sulphuric acid to the test tube.
- What do you observe? Is there a gas coming out of the delivery tube?
- Test the gas evolved successively with dry and wet blue litmus paper.
- In which case does the litmus paper change colour?
- On the basis of the above Activity, what do you infer about the acidic character of:
(i) dry HCl gas
(ii) HCl solution?
Note to teachers: If the climate is very humid, you will have to pass the gas produced through a guard tube (drying tube) containing calcium chloride to dry the gas.
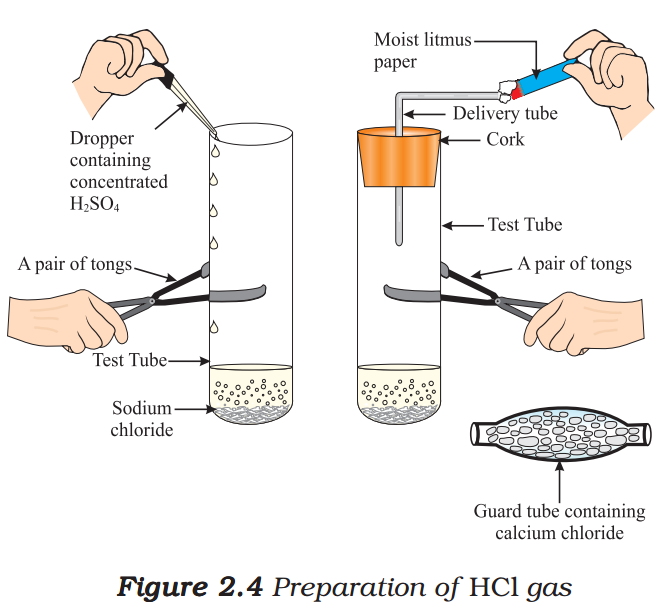
This experiment suggests that hydrogen ions in HCl are produced in the presence of water. The separation of H+ ion from HCl molecules cannot occur in the absence of water.
\( HCl+{{H}_{2}}O\to {{H}_{3}}{{O}^{+}}+C{{l}^{-}} \)
Hydrogen ions cannot exist alone, but they exist after combining with water molecules. Thus hydrogen ions must always be shown as H+(aq) or hydronium ion (H3O+).
\( {{H}^{+}}+{{H}_{2}}O\to {{H}_{3}}{{O}^{+}} \)
We have seen that acids give H3O+ or H+(aq) ion in water. Let us see what happens when a base is dissolved in water.
\( NaOH(s)\xrightarrow{{{H}_{2}}O}N{{a}^{+}}(aq)+O{{H}^{-}}(aq) \)
\( KOH(s)\xrightarrow{{{H}_{2}}O}{{K}^{+}}(aq)+O{{H}^{-}}(aq) \)
\( Mg{{(OH)}_{2}}(s)\xrightarrow{{{H}_{2}}O}M{{g}^{2+}}(aq)+2O{{H}^{-}}(aq) \)
Bases generate hydroxide (OH–) ions in water. Bases which are soluble in water are called alkalis.
Do You Know
All bases do not dissolve in water. An alkali is a base that dissolves in water. They are soapy to touch, bitter and corrosive. Never taste or touch them as they may cause harm. Which of the bases in the Table 2.1 are alkalis?
Now as we have identified that all acids generate H+(aq) and all bases generate OH–(aq), we can view the neutralisation reaction as follows –
\( Acid+Base\to Salt+Water \)
\( HX+MOH\to Mx+HOH \)
\( {{H}^{+}}(aq)+O{{H}^{-}}(aq)\leftarrow {{H}_{2}}O(\ell ) \)
Let us see what is involved when water is mixed with an acid or a base.
Activity 2.10
- Take 10 mL water in a beaker.
- Add a few drops of concentrated H2SO4 to it and swirl the beaker slowly.
- Touch the base of the beaker.
- Is there a change in temperature?
- Is this an exothermic or endothermic process?
- Repeat the above Activity with sodium hydroxide pellets and record your observations.
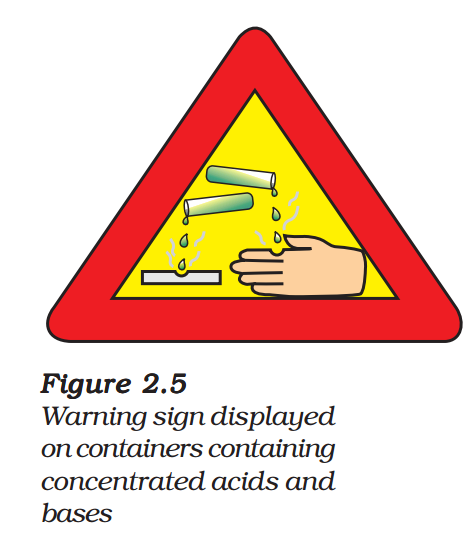
The process of dissolving an acid or a base in water is a highly exothermic one. Care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns.
The glass container may also break due to excessive local heating. Look out for the warning sign (shown in Fig. 2.5) on the can of concentrated sulphuric acid and on the bottle of sodium hydroxide pellets.
Mixing an acid or base with water results in decrease in the concentration of ions (H3O+/OH–) per unit volume. Such a process is called dilution and the acid or the base is said to be diluted.
Questions
- Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
- Why does an aqueous solution of an acid conduct electricity?
- Why does dry HCl gas not change the colour of the dry litmus paper?
- While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
- How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
- How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved in a solution of sodium hydroxide?
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

