Trung Tâm Luyện Thi Đại Học
Chapter 2. Acids Bases and Salt
2.5. Exercises - Group Actitvity
A. Exercises
1. A solution turns red litmus blue, its pH is likely to be
(a) 1 (b) 4 (c) 5 (d) 10
2. A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains
(a) NaCl (b) HCl (c) LiCl (d) KCl
3. 10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount HCl solution (the same solution as before) required to neutralise it will be
(a) 4 mL (b) 8 mL (c) 12 mL (d) 16 mL
4. Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
5. Write word equations and then balanced equations for the reaction taking place when –
(a) dilute sulphuric acid reacts with zinc granules.
(b) dilute hydrochloric acid reacts with magnesium ribbon.
(c) dilute sulphuric acid reacts with aluminium powder.
(d) dilute hydrochloric acid reacts with iron filings.
6. Compounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
7. Why does distilled water not conduct electricity, whereas rain water does?
8. Why do acids not show acidic behaviour in the absence of water?
9. Five solutions A,B,C,D and E when tested with universal indicator showed pH as
4,1,11,7 and 9, respectively. Which solution is
(a) neutral?
(b) strongly alkaline?
(c) strongly acidic?
(d) weakly acidic?
(e) weakly alkaline?
Arrange the pH in increasing order of hydrogen-ion concentration.
10. Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test
tube B. Amount and concentration taken for both the acids are same. In which test tube will the fizzing occur more vigorously and why?
11. Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
12. A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
(b) Why does this milk take a long time to set as curd?
13. Plaster of Paris should be stored in a moisture-proof container. Explain why?
14. What is a neutralisation reaction? Give two examples.
15. Give two important uses of washing soda and baking soda.
B. Group Activity
(I) Prepare your own indicator
- Crush beetroot in a mortar.
- Add sufficient water to obtain the extract.
- Filter the extract by the procedure learnt by you in earlier classes.
- Collect the filtrate to test the substances you may have tasted earlier.
- Arrange four test tubes in a test tube stand and label them as A,B,C and D. Pour 2 mL each of lemon juice solution, soda-water, vinegar and baking soda solution in them respectively.
- Put 2-3 drops of the beetroot extract in each test tube and note the colour change if any. Write your observation in a Table.
- You can prepare indicators by using other natural materials like extracts of red cabbage leaves, coloured petals of some flowers such as Petunia, Hydrangea and Geranium.
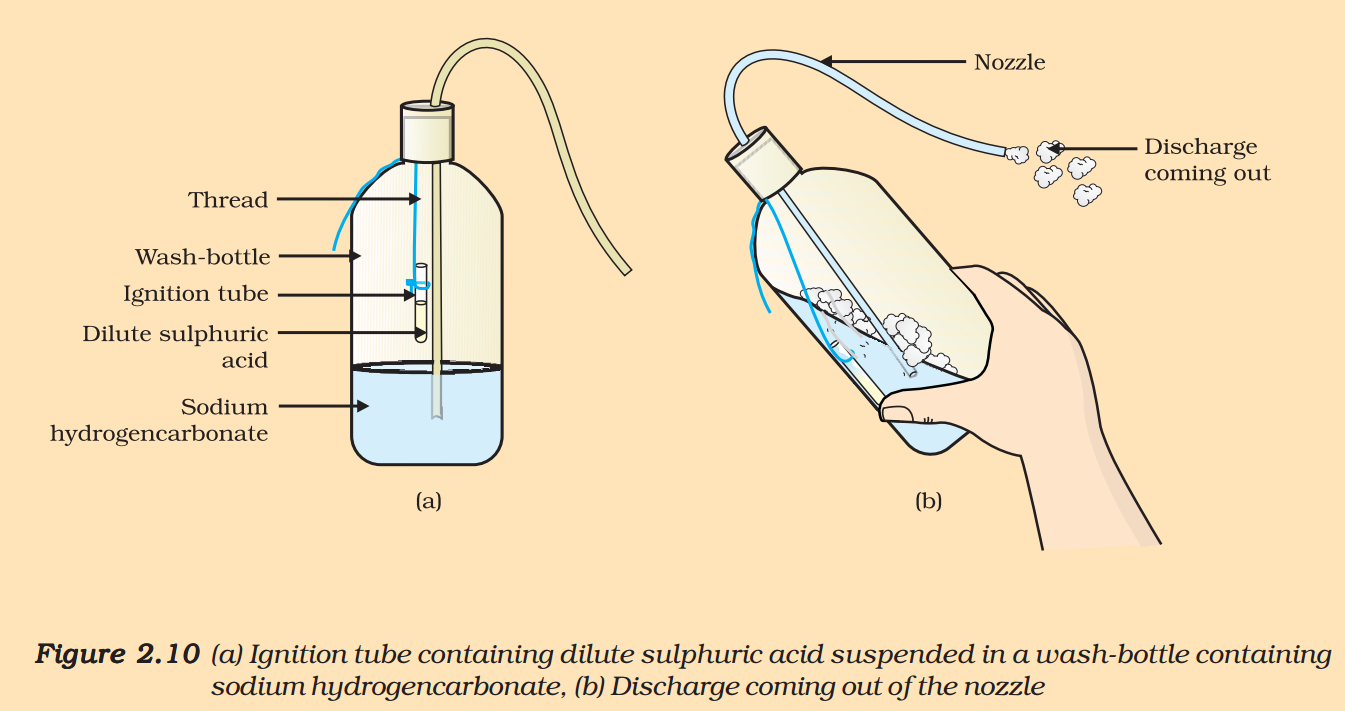
(II) Preparing a soda-acid fire extinguisher
The reaction of acids with metal hydrogencarbonates is used in the fire extinguishers which produce carbon dioxide.
- Take 20 mL of sodium hydrogencarbonate (NaHCO3) solution in a wash-bottle.
- Suspend an ignition tube containing dilute sulphuric acid in the wash-bottle (Fig. 2.10).
- Close the mouth of the wash-bottle.
- Tilt the wash-bottle so that the acid from the ignition tube mixes with the sodium hydrogencarbonate solution below.
- You will notice discharge coming out of the nozzle.
- Direct this discharge on a burning candle. What happens?
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

