Trung Tâm Luyện Thi Đại Học
Chapter 3. Metals and Non-metals
In Class IX you have lear that elements can be classified as metals or non-metals on the basis of nt about various elements. You have seen their properties.
- Think of some uses of metals and non-metals in your daily life.
- What properties did you think of while categorising elements as metals or non-metals?
- How are these properties related to the uses of these elements?
Let us look at some of these properties in detail.
3.1. Physical Properties
3.1.1 Metals
The easiest way to start grouping substances is by comparing their physical properties. Let us study this with the help of the following activities. For performing Activities 3.1 to 3.6, collect the samples of following metals – iron, copper, aluminium, magnesium, sodium, lead, zinc and any other metal that is easily available.
Activity 3.1
- Take samples of iron, copper, aluminium and magnesium. Note the appearance of each sample.
- Clean the surface of each sample by rubbing them with sand paper and note their appearance again.
Metals, in their pure state, have a shining surface. This property is called metallic lustre.
Activity 3.2
- Take small pieces of iron, copper, aluminium, and magnesium. Try to cut these metals with a sharp knife and note your observations.
- Hold a piece of sodium metal with a pair of tongs.
CAUTION: Always handle sodium metal with care. Dry it by pressing between the folds of a filter paper.
- Put it on a watch-glass and try to cut it with a knife.
- What do you observe?
You will find that metals are generally hard. The hardness varies from metal to metal.
Activity 3.3
- Take pieces of iron, zinc, lead and copper.
- Place any one metal on a block of iron and strike it four or five times with a hammer. What do you observe?
- Repeat with other metals.
- Record the change in the shape of these metals.
You will find that some metals can be beaten into thin sheets. This property is called malleability. Did you know that gold and silver are the most malleable metals?
Activity 3.4
- List the metals whose wires you have seen in daily life.
The ability of metals to be drawn into thin wires is called ductility. Gold is the most ductile metal. You will be surprised to know that a wire of about 2 km length can be drawn from one gram of gold.
It is because of their malleability and ductility that metals can be given different shapes according to our needs.
Can you name some metals that are used for making cooking vessels? Do you know why these metals are used for making vessels? Let us do the following Activity to find out the answer.
Activity 3.5
- Take an aluminium or copper wire. Clamp this wire on a stand, as shown in Fig. 3.1.
- Fix a pin to the free end of the wire using wax.
- Heat the wire with a spirit lamp, candle or a burner near the place where it is clamped.
- What do you observe after some time?
- Note your observations. Does the metal wire melt?
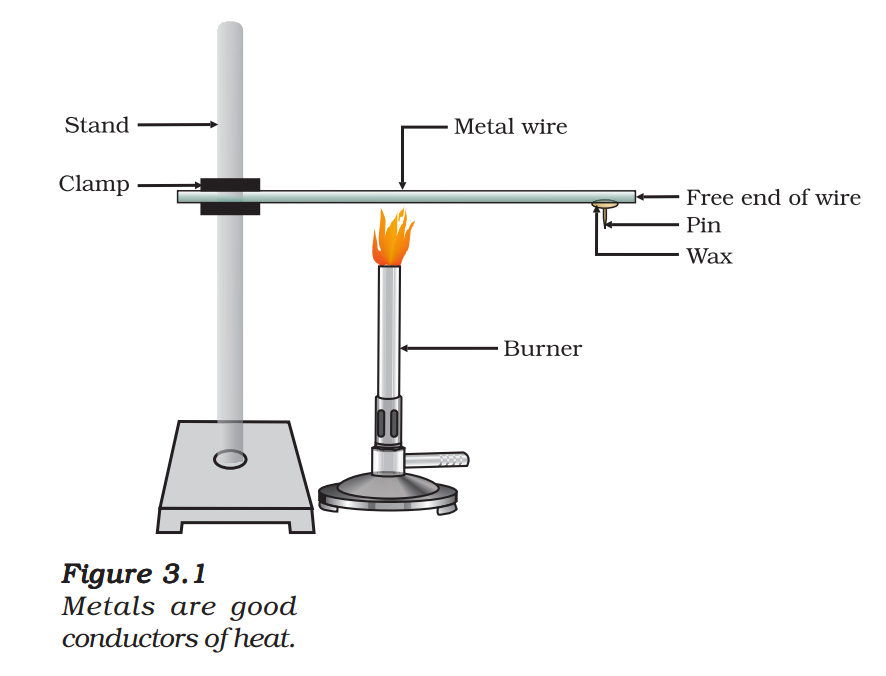
The above activity shows that metals are good conductors of heat and have high melting points. The best conductors of heat are silver and copper. Lead and mercury are comparatively poor conductors of heat.
Do metals also conduct electricity? Let us find out.
Activity 3.6
- Set up an electric circuit as shown in Fig. 3.2.
- Place the metal to be tested in the circuit between terminals A and B as shown.
- Does the bulb glow? What does this indicate?
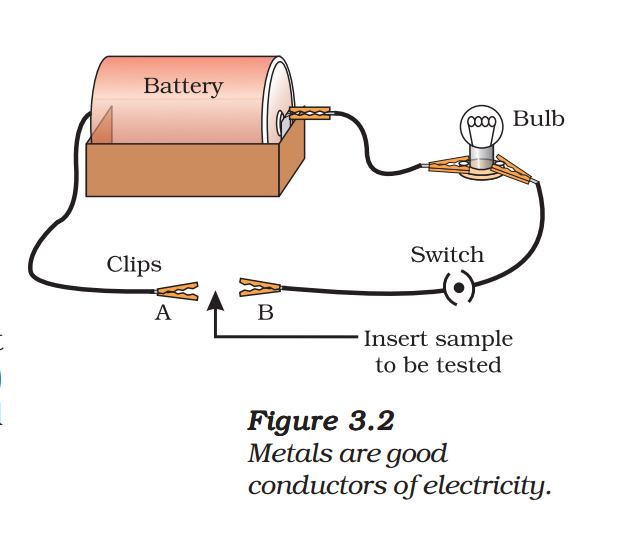
You must have seen that the wires that carry current in your homes have a coating of polyvinylchloride (PVC)
or a rubber-like material. Why are electric wires coated with such substances?
What happens when metals strike a hard surface? Do they produce a sound? The metals that produce a sound on striking a hard surface are said to be sonorous. Can you now say why school bells are made of metals?
3.1.2. Non-metals
In the previous Class you have learnt that there are very few non-metals as compared to metals. Some of the examples of non-metals are carbon, sulphur, iodine, oxygen, hydrogen, etc. The non-metals are either solids or gases except bromine which is a liquid.
Do non-metals also have physical properties similar to that of metals? Let us find out.
Activity 3.7
- Collect samples of carbon (coal or graphite), sulphur and iodine.
- Carry out the Activities 3.1 to 3.4 and 3.6 with these non-metals and record your observations.
Compile your observations regarding metals and non-metals in Table 3.1.
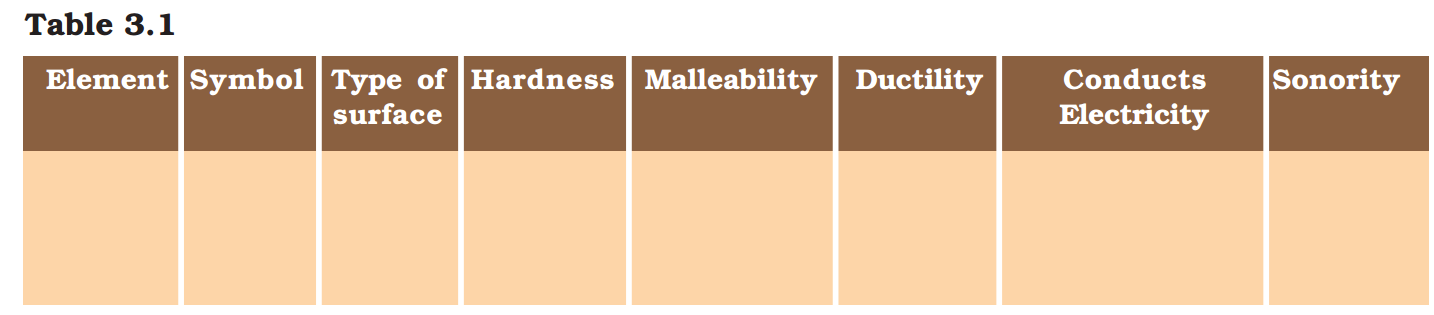
On the bases of the observations recorded in Table 3.1, discuss the general physical properties of metals and non-metals in the class. You must have concluded that we cannot group elements according to their physical properties alone, as there are many exceptions. For example –
(i) All metals except mercury exist as solids at room temperature. In Activity 3.5, you have observed that metals have high melting points but gallium and caesium have very low melting points. These two metals will melt if you keep them on your palm.
(ii) Iodine is a non-metal but it is lustrous.
(iii) Carbon is a non-metal that can exist in different forms. Each form is called an allotrope. Diamond, an allotrope of carbon, is the hardest natural substance known and has a very high melting and boiling point. Graphite, another allotrope of carbon, is a conductor of electricity.
(iv) Alkali metals (lithium, sodium, potassium) are so soft that they can be cut with a knife. They have low densities and low melting points.
Elements can be more clearly classified as metals and non-metals on the basis of their chemical properties.
Activity 3.8
- Take a magnesium ribbon and some sulphur powder.
- Burn the magnesium ribbon. Collect the ashes formed and dissolve them in water.
- Test the resultant solution with both red and blue litmus paper.
- Is the product formed on burning magnesium acidic or basic?
- Now burn sulphur powder. Place a test tube over the burning sulphur to collect the fumes produced.
- Add some water to the above test tube and shake.
- Test this solution with blue and red litmus paper.
- Is the product formed on burning sulphur acidic or basic?
- Can you write equations for these reactions?
Most non-metals produce acidic oxides when dissolve in water. On the other hand, most metals, give rise to basic oxides. You will be learning more about these metal oxides in the next section.
Questions
1. Give an example of a metal which
(i) is a liquid at room temperature.
(ii) can be easily cut with a knife.
(iii) is the best conductor of heat.
(iv) is a poor conductor of heat.
2. Explain the meanings of malleable and ductile.
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

