Trung Tâm Luyện Thi Đại Học
Chapter 1. Chemical Reactions and Equations
Consider the following situations of daily life and think what happens when –
- milk is left at room temperature during summers.
- an iron tawa/pan/nail is left exposed to humid atmosphere.
- grapes get fermented.
- food is cooked.
- food gets digested in our body.
- we respire.
In all the above situations, the nature and the identity of the initial substance have somewhat changed. We have already learnt about physical and chemical changes of matter in our previous classes. Whenever a chemical change occurs, we can say that a chemical reaction has taken place.
You may perhaps be wondering as to what is actually meant by a chemical reaction. How do we come to know that a chemical reaction has taken place? Let us perform some activities to find the answer to these questions.
Activity 1.1
CAUTION: This Activity needs the teacher’s assistance. It would be better if students wear suitable eyeglasses.
- Clean a magnesium ribbon about 3-4 cm long by rubbing it with sandpaper.
- Hold it with a pair of tongs. Burn it using a spirit lamp or burner and collect the ash so formed in a watch-glass as shown in Fig. 1.1. Burn the magnesium ribbon keeping it away as far as possible from your eyes.
- What do you observe?
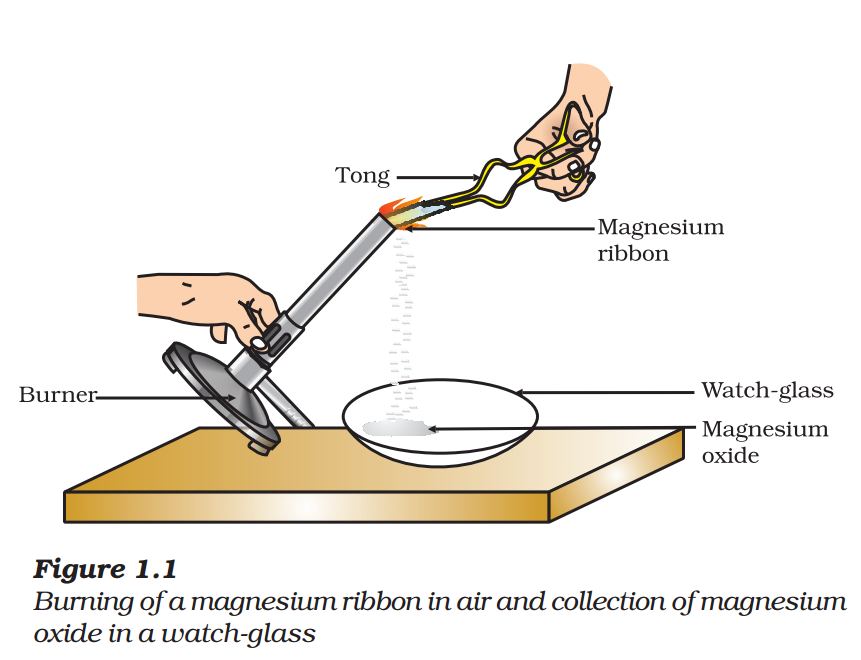
You must have observed that magnesium ribbon burns with a dazzling white flame and changes into a white powder. This powder is magnesium oxide. It is formed due to the reaction between magnesium and oxygen present in the air.
Activity 1.2
- Take lead nitrate solution in a test tube.
- Add potassium iodide solution to this.
- What do you observe?
Activity 1.3
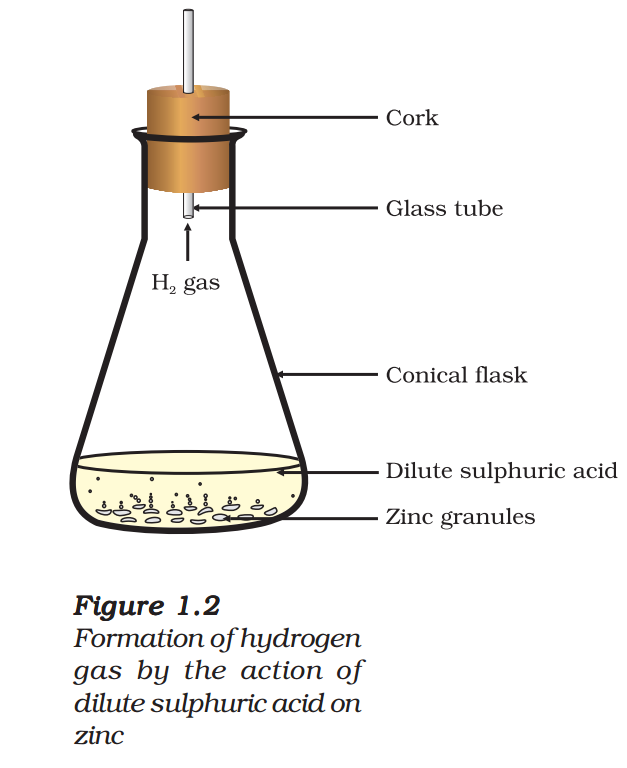
- Take a few zinc granules in a conical flask or a test tube.
- Add dilute hydrochloric acid or sulphuric acid to this (Fig. 1.2).
CAUTION: Handle the acid with care. - Do you observe anything happening around the zinc granules?
- Touch the conical flask or test tube. Is there any change in its temperature?
From the above three activities, we can say that any of the following observations helps us to determine whether a chemical reaction has taken place –
- change in state
- change in colour
- evolution of a gas
- change in temperature.
As we observe the changes around us, we can see that there is a large variety of chemical reactions taking place around us. We will study about the various types of chemical reactions and their symbolic representation in this Chapter.
1.1. Chemical equations
Activity 1.1 can be described as – when a magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide. This description of a chemical reaction in a sentence form is quite long. It can be written in a shorter form. The simplest way to do this is to write it in the form of a word-equation.
The word-equation for the above reaction would be –
\( \begin{align} & Magnesium+Oxygen\to Magnesium\,\,oxide\,\,\,\,\,\,\,\,\,\,\,\,(1.1) \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{Reactants})\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{Product}) \\ \end{align} \)
The substances that undergo chemical change in the reaction (1.1), magnesium and oxygen, are the reactants. The new substance is magnesium oxide, formed during the reaction, as a product.
A word-equation shows change of reactants to products through an arrow placed between them. The reactants are written on the left-hand side (LHS) with a plus sign (+) between them. Similarly, products are written on the right-hand side (RHS) with a plus sign (+) between them. The arrowhead points towards the products, and shows the direction of the reaction.
1.1.1 Writing a Chemical Equation
Is there any other shorter way for representing chemical equations?
Chemical equations can be made more concise and useful if we use chemical formulae instead of words. A chemical equation represents a chemical reaction. If you recall formulae of magnesium, oxygen and magnesium oxide, the above word-equation can be written as –
\( Mg+{{O}_{2}}\to MgO\,\,\,\,\,\,(1.2) \)
Count and compare the number of atoms of each element on the LHS and RHS of the arrow. Is the number of atoms of each element the same on both the sides? If yes, then the equation is balanced. If not, then the equation is unbalanced because the mass is not the same on both sides of the equation. Such a chemical equation is a skeletal chemical equation for a reaction. Equation (1.2) is a skeletal chemical equation for the burning of magnesium in air.
1.1.2 Balanced Chemical Equations
Recall the law of conservation of mass that you studied in Class IX; mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction
has to be equal to the total mass of the elements present in the reactants.
In other words, the number of atoms of each element remains the same, before and after a chemical reaction. Hence, we need to balance a skeletal chemical equation. Is the chemical Eq. (1.2) balanced? Let us learn about balancing a chemical equation step by step.
The word-equation for Activity 1.3 may be represented as –
\( Zinc+Sulphuric\,\,acid\to Zinc\,\,sulphate+Hydrogen \)
The above word-equation may be represented by the following chemical equation –
\( Zn+{{H}_{2}}S{{O}_{4}}\to ZnS{{O}_{4}}+{{H}_{2}}\,\,\,\,\,\,\,(1.3) \)
Let us examine the number of atoms of different elements on both sides of the arrow.
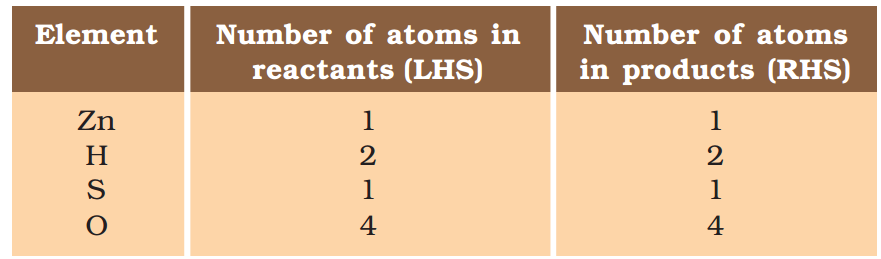
As the number of atoms of each element is the same on both sides of the arrow, Eq. (1.3) is a balanced chemical equation.
Let us try to balance the following chemical equation –
\( Fe+{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+{{H}_{2}}\,\,\,\,\,\,\,(1.4) \)
Step I: To balance a chemical equation, first draw boxes around each formula. Do not change anything inside the boxes while balancing the equation.
\( Fe+{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+{{H}_{2}}\,\,\,\,\,\,\,(1.5) \)
Step II: List the number of atoms of different elements present in the unbalanced equation (1.5).
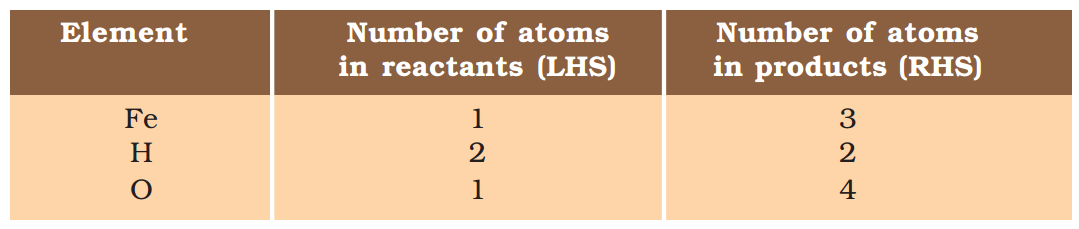
Step III: It is often convenient to start balancing with the compound that contains the maximum number of atoms. It may be a reactant or a product. In that compound, select the element which has the maximum number of atoms. Using these criteria, we select Fe3O4 and the element oxygen in it. There are four oxygen atoms on the RHS and only one on the LHS.
To balance the oxygen atoms –
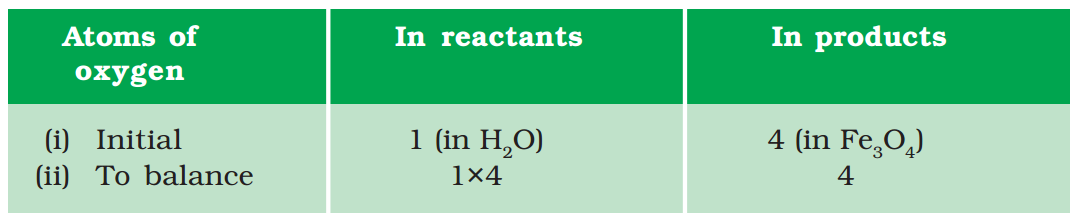
To equalise the number of atoms, it must be remembered that we cannot alter the formulae of the compounds or elements involved in the reactions. For example, to balance oxygen atoms we can put coefficient ‘4’ as 4 H2O and not H2O4 or (H2O)4. Now the partly balanced equation becomes –
\( Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+{{H}_{2}}\,\,\,\,\,\,\,\,(1.6)\,\,(partly\,\,balanced\,\,equation) \)
Step IV: Fe and H atoms are still not balanced. Pick any of these elements to proceed further. Let us balance hydrogen atoms in the partly balanced equation.
To equalise the number of H atoms, make the number of molecules of hydrogen as four on the RHS.

The equation would be –
\( Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\,\,\,\,\,\,\,\,\,(1.7)\,\,(partly\,\,banlanced\,\,equation) \)
Step V: Examine the above equation and pick up the third element which is not balanced. You find that only one element is left to be balanced, that is, iron.
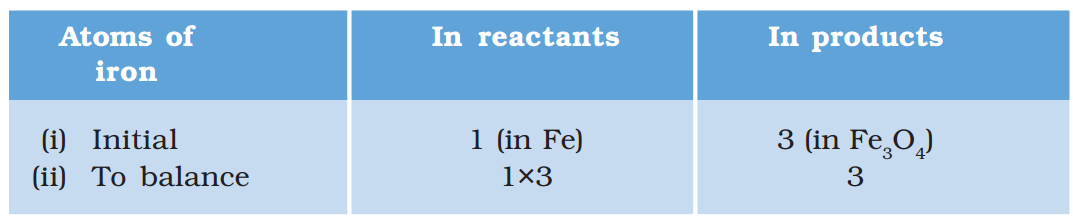
To equalise Fe, we take three atoms of Fe on the LHS.
\( 3Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\,\,\,\,\,\,\,\,(1.8) \)
Step VI: Finally, to check the correctness of the balanced equation, we count atoms of each element on both sides of the equation.
\( 3Fe+4{{H}_{2}}O\to F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\,\,\,\,\,\,\,\,\,(1.9)\,\,(balanced\,\,equation) \)
The numbers of atoms of elements on both sides of Eq. (1.9) are equal. This equation is now balanced. This method of balancing chemical equations is called hit-and-trial method as we make trials to balance the equation by using the smallest whole number coefficient.
Step VII: Writing Symbols of Physical States Carefully examine the above balanced Eq. (1.9). Does this equation tell us anything about the physical state of each reactant and product? No information has been given in this equation about their physical states.
To make a chemical equation more informative, the physical states of the reactants and products are mentioned along with their chemical formulae. The gaseous, liquid, aqueous and solid states of reactants
and products are represented by the notations (g), (l), (aq) and (s), respectively. The word aqueous (aq) is written if the reactant or product is present as a solution in water.
The balanced Eq. (1.9) becomes
\( 3Fe(s)+4{{H}_{2}}O(g)\to F{{e}_{3}}{{O}_{4}}(s)+4{{H}_{2}}(g)\,\,\,\,\,(1.10) \)
Note that the symbol (g) is used with H2O to indicate that in this reaction water is used in the form of steam.
Usually physical states are not included in a chemical equation unless it is necessary to specify them.
Sometimes the reaction conditions, such as temperature, pressure, catalyst, etc., for the reaction are indicated above and or below the arrow in the equation. For example
\( CO(g)+2{{H}_{2}}(g)\xrightarrow{340\,atm}C{{H}_{3}}OH(\ell )\,\,\,\,\,\,\,(1.11) \)
\( \begin{align} & 6C{{O}_{2}}(aq)+12{{H}_{2}}O(\ell )\xrightarrow[Chlorophyll]{Sunlight}{{C}_{6}}{{H}_{12}}{{O}_{6}}(aq)+6{{O}_{2}}(aq)+6{{H}_{2}}O(\ell )\,\,\,\,\,\,\,(1.12) \\ &\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{glucose}) \\ \end{align} \)
Using these steps, can you balance Eq. (1.2) given in the text earlier?
Questions
1. Why should a magnesium ribbon be cleaned before burning in air?
2. Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine → Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
(iii) Sodium + Water → Sodium hydroxide + Hydrogen
3. Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

