Trung Tâm Luyện Thi Đại Học
Chapter 3. Metals and Non-metals
3.3. How Do Metals And Non-Metals React?
In the above activities, you saw the reactions of metals with a number of reagents. Why do metals react in this manner? Let us recall what we learnt about the electronic configuration of elements in Class IX. We learnt that noble gases, which have a completely filled valence shell, show little chemical activity. We, therefore, explain the reactivity of elements as a tendency to attain a completely filled valence shell.
Let us have a look at the electronic configuration of noble gases and some metals and non-metals.
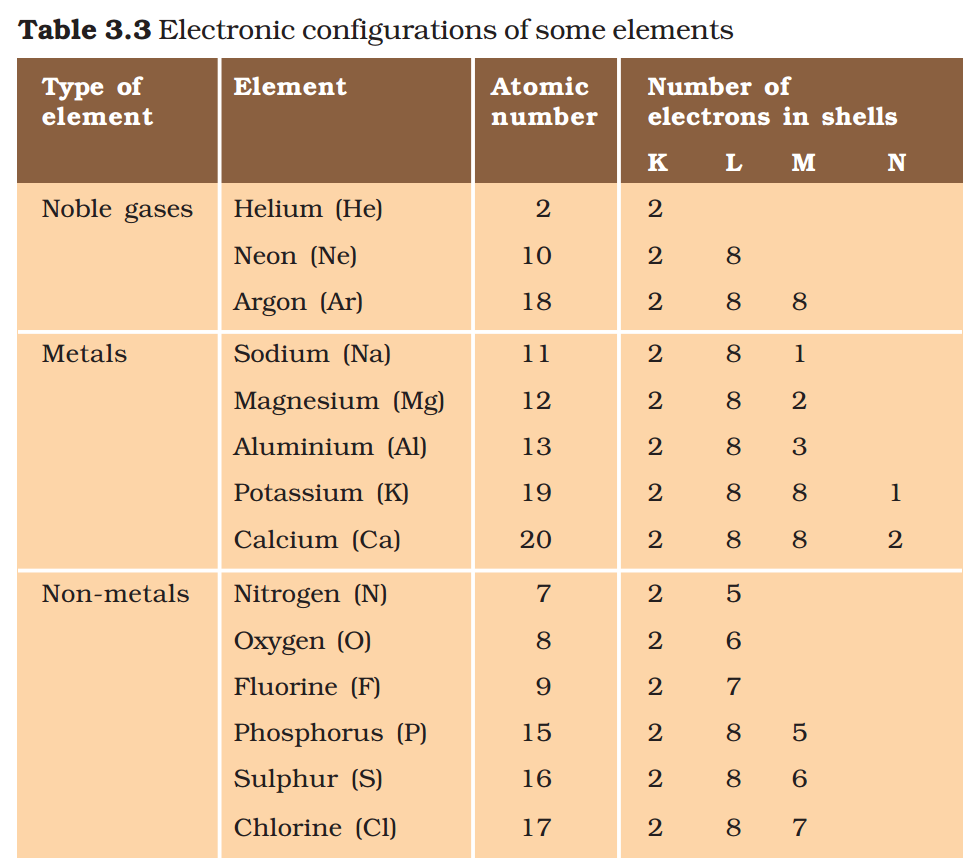
We can see from Table 3.3 that a sodium atom has one electron in its outermost shell. If it loses the electron from its M shell then its L shell now becomes the outermost shell and that has a stable octet. The nucleus of this atom still has 11 protons but the number of electrons has become 10, so there is a net positive charge giving us a sodium cation Na+. On the other hand chlorine has seven electrons in its outermost shell and it requires one more electron to complete its octet. If sodium and chlorine were to react, the electron lost by sodium could be taken up by chlorine.
After gaining an electron, the chlorine atom gets a unit negative charge, because its nucleus has 17 protons and there are 18 electrons in its K, L and M shells. This gives us a chloride anion C1–. So both these elements can have a give-and-take relation between them as follows (Fig. 3.5).
\( \begin{align} & Na\,\,\,\,\,\to \,\,\,\,N{{a}^{+}}+{{e}^{-}} \\ & 2,8,1\,\,\,\,\,\,\,\,\,\,\,2,8\,\,(Sodium\,\,cation) \\ \end{align} \)
\( \begin{align} & Cl\,\,\,\,\,+{{e}^{-}}\to \,\,\,\,C{{l}^{-}} \\ & 2,8,7\,\,\,\,\,\,\,\,\,\,\,2,8,8\,\,(Chloride\,\,ation) \\ \end{align} \)
Sodium and chloride ions, being oppositely charged, attract each other and are held by strong electrostatic forces of attraction to exist as sodium chloride (NaCl). It should be noted that sodium chloride does not exist as molecules but aggregates of oppositely charged ions.
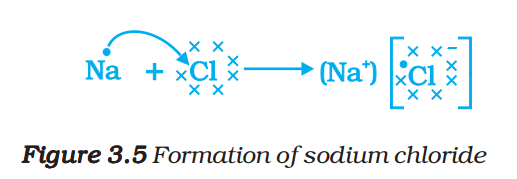
Let us see the formation of one more ionic compound, magnesium chloride (Fig. 3.6).
The compounds formed in this manner by the transfer of electrons from a metal to a non-metal are known as ionic compounds or electrovalent compounds. Can you name the cation and anion present in MgCl2?
3.3.1 Properties of Ionic Compounds
To learn about the properties of ionic compounds, let us perform the following Activity:
Activity 3.13
- Take samples of sodium chloride, potassium iodide, barium chloride or any other salt from the science laboratory.
- What is the physical state of these salts?
- Take a small amount of a sample on a metal spatula and heat directly on the flame (Fig. 3.7). Repeat with other samples.
- What did you observe? Did the samples impart any colour to the flame? Do these compounds melt?
- Try to dissolve the samples in water, petrol and kerosene. Are they soluble?
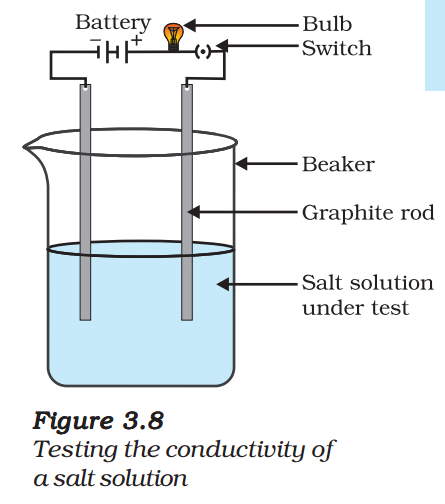
- Make a circuit as shown in Fig. 3.8 and insert the electrodes into a solution of one salt. What did you observe? Test the other salt samples too in this manner.
- What is your inference about the nature of these compounds?
You may have observed the following general properties for ionic
compounds—
(i) Physical nature: Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions. These compounds are generally brittle and break into pieces when pressure is applied.
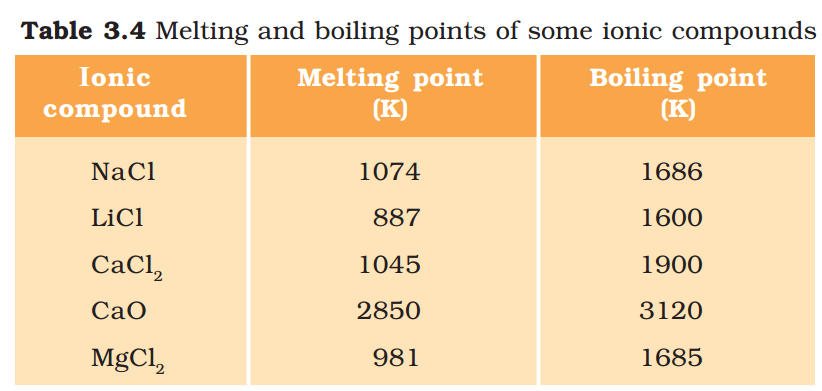
(ii) Melting and Boiling points: Ionic compounds have high melting and boiling points (see Table 3.4). This is because a considerable amount of energy is required to break the strong inter-ionic attraction.
(iii) Solubility: Electrovalent compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
(iv) Conduction of Electricity: The conduction of electricity through a solution involves the movement of charged particles. A solution of an ionic compound in water contains ions, which move to the opposite electrodes when electricity is passed through the solution. Ionic compounds in the solid state do not conduct electricity because movement of ions in the solid is not possible due to their rigid structure. But ionic compounds conduct electricity in the molten state. This is possible in the molten state since the elecrostatic forces of attraction between the oppositely charged ions are overcome due to the heat. Thus, the ions move freely and conduct electricity.
Questions
1.
(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
2. Why do ionic compounds have high melting points?
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

