Trung Tâm Luyện Thi Đại Học
Chapter 3. Metals and Non-metals
3.4. Occurrence Of Metals
The earth’s crust is the major source of metals. Seawater also contains some soluble salts such as sodium chloride, magnesium chloride, etc.
The elements or compounds, which occur naturally in the earth’s crust, are known as minerals. At some places, minerals contain a very high percentage of a particular metal and the metal can be profitably extracted from it. These minerals are called ores.
3.4.1 Extraction of Metals
You have learnt about the reactivity series of metals. Having this knowledge, you can easily understand how a metal is extracted from its ore. Some metals are found in the earth’s crust in the free state. Some are found in the form of their compounds. The metals at the bottom of the activity series are the least reactive. They are often found in a free state. For example, gold, silver, platinum and copper are found in the free state. Copper and silver are also found in the combined state as their sulphide or oxide ores. The metals at the top of the activity series (K, Na, Ca, Mg and Al) are so reactive that they are never found in nature as free elements. The metals in the middle of the activity series (Zn, Fe, Pb, etc.) are moderately reactive. They are found in the earth’s crust mainly as oxides, sulphides or carbonates. You will find that the ores of many metals are oxides. This is because oxygen is a very reactive element and is very abundant on the earth.
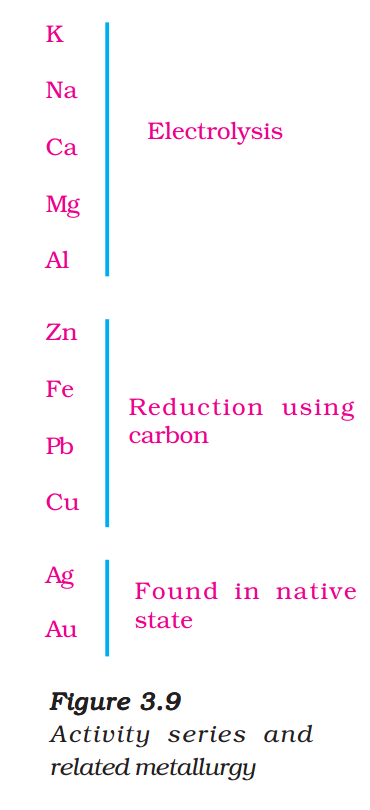
Thus on the basis of reactivity, we can group the metals into the following three categories (Fig. 3.9) – (i) Metals of low reactivity; (ii) Metals of medium reactivity; (iii) Metals of high reactivity. Different techniques are to be used for obtaining the metals falling in each category.
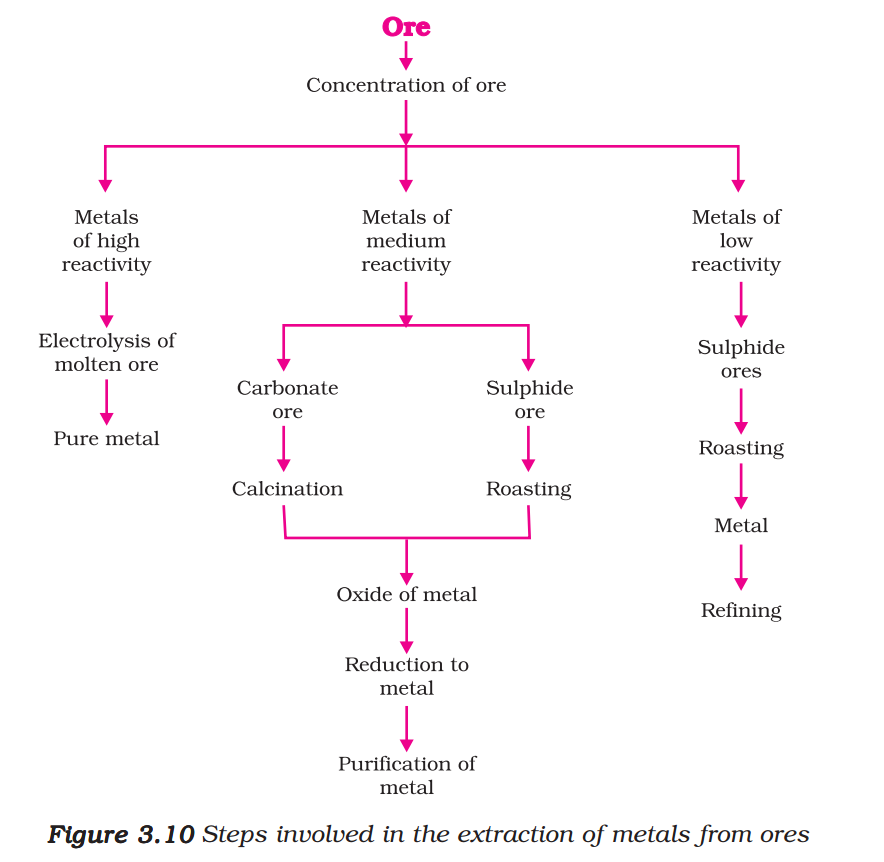
Several steps are involved in the extraction of pure metal from ores. A summary of these steps is given in Fig.3.10. Each step is explained in detail in the following sections.
3.4.2 Enrichment of Ores
Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue. The impurities must be removed from the ore prior to the extraction of the metal. The processes used for removing the gangue from the ore are based on the differences between the physical or chemical properties of the gangue and the ore. Different separation techniques are accordingly employed.
3.4.3 Extracting Metals Low in the Activity Series
Metals low in the activity series are very unreactive. The oxides of these metals can be reduced to metals by heating alone. For example, cinnabar (HgS) is an ore of mercury. When it is heated in air, it is first converted
into mercuric oxide (HgO). Mercuric oxide is then reduced to mercury on further heating.
\( 2HgS(s)+3{{O}_{2}}(g)\xrightarrow{Heat}2HgO(s)+2S{{O}_{2}}(g) \)
\( 2HgO(s)\xrightarrow{Heat}2Hg(\ell )+{{O}_{2}}(g) \)
Similarly, copper which is found as Cu2S in nature can be obtained from its ore by just heating in air.
\( 2C{{u}_{2}}S+3{{O}_{2}}(g)\xrightarrow{Heat}2C{{u}_{2}}O(s)+2S{{O}_{2}}(g) \)
\( 2C{{u}_{2}}O+C{{u}_{2}}S\xrightarrow{Heat}6Cu(s)+2S{{O}_{2}}(g) \)
3.4.4 Extracting Metals in the Middle of the Activity Series
The metals in the middle of the activity series such as iron, zinc, lead, copper, are moderately reactive. These are usually present as sulphides or carbonates in nature. It is easier to obtain a metal from its oxide, as compared to its sulphides and carbonates. Therefore, prior to reduction, the metal sulphides and carbonates must be converted into metal oxides. The sulphide ores are converted into oxides by heating strongly in the presence of excess air. This process is known as roasting. The carbonate ores are changed into oxides by heating strongly in limited air. This process is known as calcination. The chemical reaction that takes place during roasting and calcination of zinc ores can be shown as follows –
Roasting
\( 2ZnS(s)+3{{O}_{2}}(g)\xrightarrow{Heat}2ZnO(s)+2S{{O}_{2}}(g) \)
Calcination
\( ZnC{{O}_{3}}(s)\xrightarrow{Heat}ZnO(s)+C{{O}_{2}}(g) \)
The metal oxides are then reduced to the corresponding metals by using suitable reducing agents such as carbon. For example, when zinc oxide is heated with carbon, it is reduced to metallic zinc.
\( ZnO(s)+C(s)\to Zn(s)+CO(g) \)
You are already familiar with the process of oxidation and reduction explained in the first Chapter. Obtaining metals from their compounds is also a reduction process.
Besides using carbon (coke) to reduce metal oxides to metals, sometimes displacement reactions can also be used. The highly reactive metals such as sodium, calcium, aluminium, etc., are used as reducing agents because they can displace metals of lower reactivity from their compounds. For example, when manganese dioxide is heated with aluminium powder, the following reaction takes place –
\( 3Mn{{O}_{2}}(s)+4Al(s)\to 3Mn(\ell )+2A{{l}_{2}}{{O}_{3}}(s)+Heat \)
Can you identify the substances that are getting oxidised and reduced?
These displacement reactions are highly exothermic. The amount of heat evolved is so large that the metals are produced in the molten state. In fact, the reaction of iron(III) oxide (Fe2O3) with aluminium is used to join railway tracks or cracked machine parts. This reaction is known as the thermit reaction.
\( F{{e}_{2}}{{O}_{3}}(s)+2Al(s)\to 2Fe(\ell )+A{{l}_{2}}{{O}_{3}}(s)+Heat \)
3.4.5 Extracting Metals towards the Top of the Activity Series
The metals high up in the reactivity series are very reactive. They cannot be obtained from their compounds by heating with carbon. For example, carbon cannot reduce the oxides of sodium, magnesium, calcium, aluminium, etc., to the respective metals. This is because these metals have more affinity for oxygen than carbon. These metals are obtained by electrolytic reduction. For example, sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides. The metals are deposited at the cathode (the negatively charged electrode), whereas, chlorine is liberated at the anode (the positively charged electrode). The reactions are –
At cathode: \( N{{a}^{+}}+{{e}^{-}}\to Na \)
At anode: \( 2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}} \)
Similarly, aluminium is obtained by the electrolytic reduction of aluminium oxide.
3.4.6 Refining of Metals
The metals produced by various reduction processes described above are not very pure. They contain impurities, which must be removed to obtain pure metals. The most widely used method for refining impure metals is electrolytic refining.
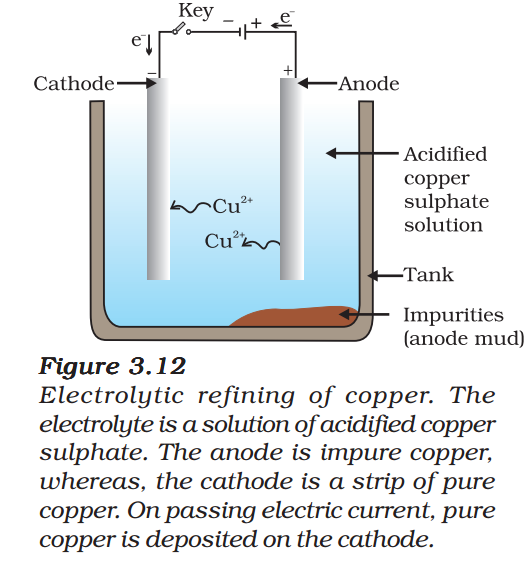
Electrolytic Refining: Many metals, such as copper, zinc, tin, nickel, silver, gold, etc., are refined electrolytically. In this process, the impure metal is made the anode and a thin strip of pure metal is made the cathode. A solution of the metal salt is used as an electrolyte. The apparatus is set up as shown in Fig. 3.12. On passing the current through the electrolyte, the pure metal from the anode dissolves into the electrolyte. An equivalent amount of pure metal from the electrolyte is deposited on the cathode. The soluble impurities go into the solution, whereas, the insoluble impurities settle down at the bottom of the anode and are known as anode mud.
Questions
1. Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
2. Name two metals which are found in nature in the free state.
3. What chemical process is used for obtaining a metal from its oxide?
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

