Trung Tâm Luyện Thi Đại Học
Chapter 3. Metals and Non-metals
3.2. Chemical Properties Of Metals
We will learn about the chemical properties of metals in the following Sections 3.2.1 to 3.2.4. For this, collect the samples of following metals – aluminium, copper, iron, lead, magnesium, zinc and sodium.
3.2.1. What happens when Metals are burnt in Air?
You have seen in Activity 3.8 that magnesium burns in air with a dazzling white flame. Do all metals react in the same manner? Let us check by performing the following Activity.
Activity 3.9
CAUTION: The following activity needs the teacher’s assistance. It would be better if students wear eye protection.
- Hold any of the samples taken above with a pair of tongs and try burning over a flame. Repeat with the other metal samples.
- Collect the product if formed.
- Let the products and the metal surface cool down.
- Which metals burn easily?
- What flame colour did you observe when the metal burnt?
- How does the metal surface appear after burning?
- Arrange the metals in the decreasing order of their reactivity towards oxygen.
- Are the products soluble in water?
Almost all metals combine with oxygen to form metal oxides.
Metal + Oxygen → Metal oxide
For example, when copper is heated in air, it combines with oxygen to form copper(II) oxide, a black oxide.
\( \begin{align} & 2Cu+{{O}_{2}}\to 2CuO \\ & (Copper)\,\,\,\,\,\,\,\,\,(Copper(II)\,\,oxide) \\ \end{align} \)
Similarly, aluminium forms aluminium oxide.
\( \begin{align} & 4Al\,\,\,\,\,\,\,\,\,\,\,+\,\,\,\,\,\,\,\,\,3{{O}_{2}}\to 2A{{l}_{2}}{{O}_{3}} \\ &(\text{Aluminium})\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{Aluminium}\,\,\text{oxide}) \\ \end{align} \)
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We have learnt that metal oxides are basic in nature. But some metal oxides, such as aluminium oxide, zinc oxide show both acidic as well as basic behaviour. Such metal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides. Aluminium oxide reacts in the following manner with acids and bases –
\( A{{l}_{2}}{{O}_{3}}+6HCl\to 2AlC{{l}_{3}}+3{{H}_{2}}O \)
\( \begin{align} & A{{l}_{2}}{{O}_{3}}+2NaOH\to 2NaAl{{O}_{2}}+{{H}_{2}}O \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{Sodium}\,\,\text{aluminate}) \\ \end{align} \)
Most metal oxides are insoluble in water but some of these dissolve in water to form alkalis. Sodium oxide and potassium oxide dissolve in water to produce alkalis as follows –
\( N{{a}_{2}}O(s)+{{H}_{2}}O(\ell )\to 2NaOH(aq) \)
\( {{K}_{2}}O(s)+{{H}_{2}}O(\ell )\to 2KOH(aq) \)
We have observed in Activity 3.9 that all metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen. Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
At ordinary temperature, the surfaces of metals such as magnesium, aluminium, zinc, lead, etc., are covered with a thin layer of oxide. The protective oxide layer prevents the metal from further oxidation. Iron does not burn on heating but iron filings burn vigorously when sprinkled in the flame of the burner. Copper does not burn, but the hot metal is coated with a black coloured layer of copper(II) oxide. Silver and gold do not react with oxygen even at high temperatures.
Do You Know?
Anodising is a process of forming a thick oxide layer of aluminium. Aluminium develops a thin oxide layer when exposed to air. This aluminium oxide coat makes it resistant to further corrosion. The resistance can be improved further by making the oxide layer thicker. During anodising, a clean aluminium article is made the anode and is electrolysed with dilute sulphuric acid. The oxygen gas evolved at the anode reacts with aluminium to make a thicker protective oxide layer. This oxide layer can be dyed easily to give aluminium articles an attractive finish.
After performing Activity 3.9, you must have observed that sodium is the most reactive of the samples of metals taken here. The reaction of magnesium is less vigorous implying that it is not as reactive as sodium.
But burning in oxygen does not help us to decide about the reactivity of zinc, iron, copper or lead. Let us see some more reactions to arrive at a conclusion about the order of reactivity of these metals.
3.2.2. What happens when Metals react with Water?
Activity 3.10
CAUTION: This Activity needs the teacher’s assistance.
- Collect the samples of the same metals as in Activity 3.9.
- Put small pieces of the samples separately in beakers half-filled with cold water.
- Which metals reacted with cold water? Arrange them in the increasing order of their reactivity with cold water.
- Did any metal produce fire on water?
- Does any metal start floating after some time?
- Put the metals that did not react with cold water in beakers half-filled with hot water.
- For the metals that did not react with hot water, arrange the apparatus as shown in Fig. 3.3 and observe their reaction with steam.
- Which metals did not react even with steam?
- Arrange the metals in the decreasing order of reactivity with water.
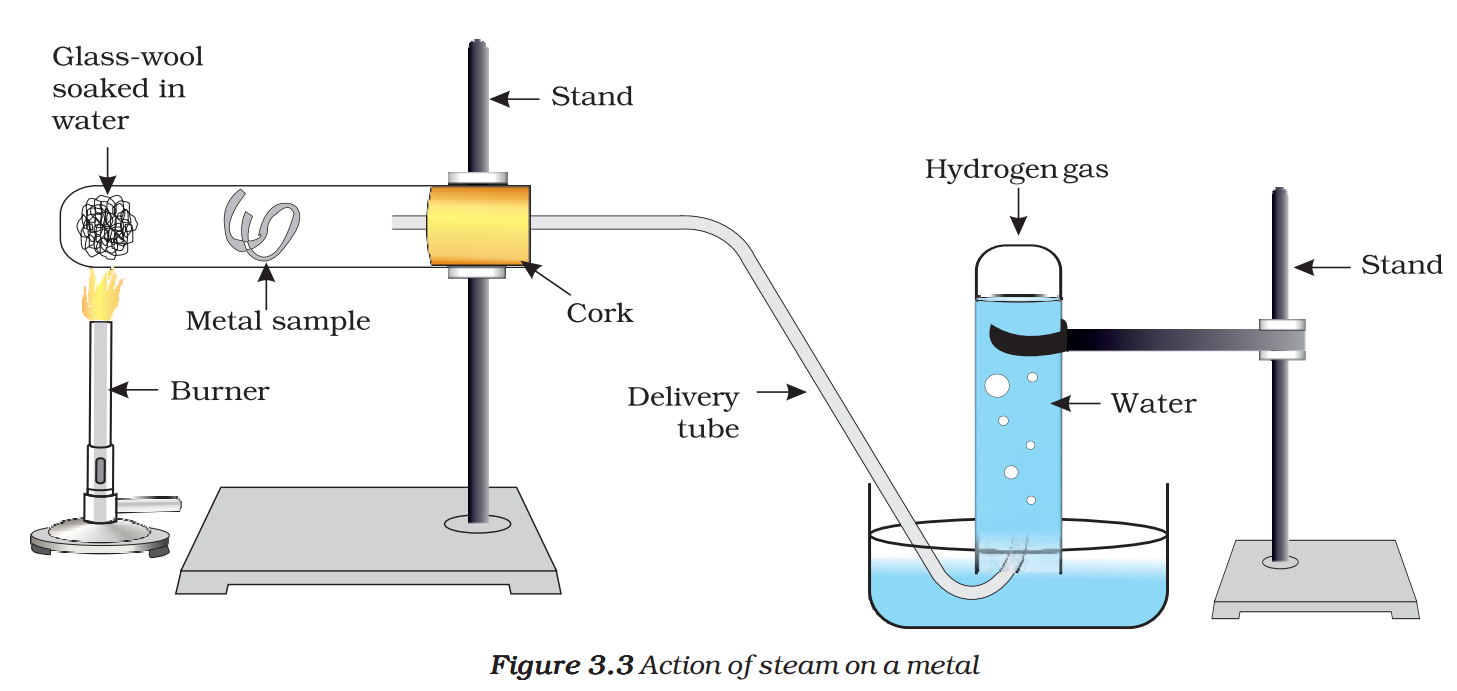
Metals react with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal hydroxide. But all metals do not react with water.
Metal + Water → Metal oxide + Hydrogen
Metal oxide + Water → Metal hydroxide
Metals like potassium and sodium react violently with cold water. In case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
\( 2K(s)+2{{H}_{2}}O(\ell )\to 2KOH(aq)+{{H}_{2}}(g)+heat\,\,energy \)
\( 2Na(s)+2{{H}_{2}}O(\ell )\to 2NaOH(aq)+{{H}_{2}}(g)+heat\,\,energy \)
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
\( Ca(s)+2{{H}_{2}}O(\ell )\to Ca{{(OH)}_{2}}(aq)+{{H}_{2}}(g) \)
Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen. It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
Metals like aluminium, iron and zinc do not react either with cold or hot water. But they react with steam to form the metal oxide and hydrogen.
\( 2Al(s)+3{{H}_{2}}O(g)\to A{{l}_{2}}{{O}_{3}}(s)+3{{H}_{2}}(g) \)
\( 3Fe(s)+4{{H}_{2}}O(g)\to F{{e}_{3}}{{O}_{4}}(s)+4{{H}_{2}}(g) \)
Metals such as lead, copper, silver and gold do not react with water at all.
3.2.3. What happens when Metals react with Acids?
You have already learnt that metals react with acids to give a salt and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
But do all metals react in the same manner? Let us find out.
Activity 3.11
- Collect all the metal samples except sodium and potassium again.
If the samples are tarnished, rub them clean with sand paper.
CAUTION: Do not take sodium and potassium as they react vigorously even with cold water. - Put the samples separately in test tubes containing dilute hydrochloric acid.
- Suspend thermometers in the test tubes, so that their bulbs are dipped in the acid.
- Observe the rate of formation of bubbles carefully.
- Which metals reacted vigorously with dilute hydrochloric acid?
- With which metal did you record the highest temperature?
- Arrange the metals in the decreasing order of reactivity with dilute acids.
Write equations for the reactions of magnesium, aluminium, zinc and iron with dilute hydrochloric acid.
Hydrogen gas is not evolved when a metal reacts with nitric acid. It is because HNO3 is a strong oxidising agent. It oxidises the H2 produced to water and itself gets reduced to any of the nitrogen oxides (N2O, NO, NO2). But magnesium (Mg) and manganese (Mn) react with very dilute HNO3 to evolve H2 gas.
You must have observed in Activity 3.11, that the rate of formation of bubbles was the fastest in the case of magnesium. The reaction was also the most exothermic in this case. The reactivity decreases in the order Mg > Al > Zn > Fe. In the case of copper, no bubbles were seen and the temperature also remained unchanged. This shows that copper does not react with dilute HCl.
Do You Know?
Aqua regia, (Latin for ‘royal water’) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1. It can dissolve gold, even though neither of these acids can do so alone. Aqua regia is a highly corrosive, fuming liquid. It is one of the few reagents that is able to dissolve gold and platinum.
3.2.4 How do Metals react with Solutions of other Metal Salts?
Activity 3.12
- Take a clean wire of copper and an iron nail.
- Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in test tubes (Fig. 3.4).
- Record your observations after 20 minutes.
- In which test tube did you find that a reaction has occurred?
- On what basis can you say that a reaction has actually taken place?
- Can you correlate your observations for the Activities 3.9, 3.10 and 3.11?
- Write a balanced chemical equation for the reaction that has taken place.
- Name the type of reaction.
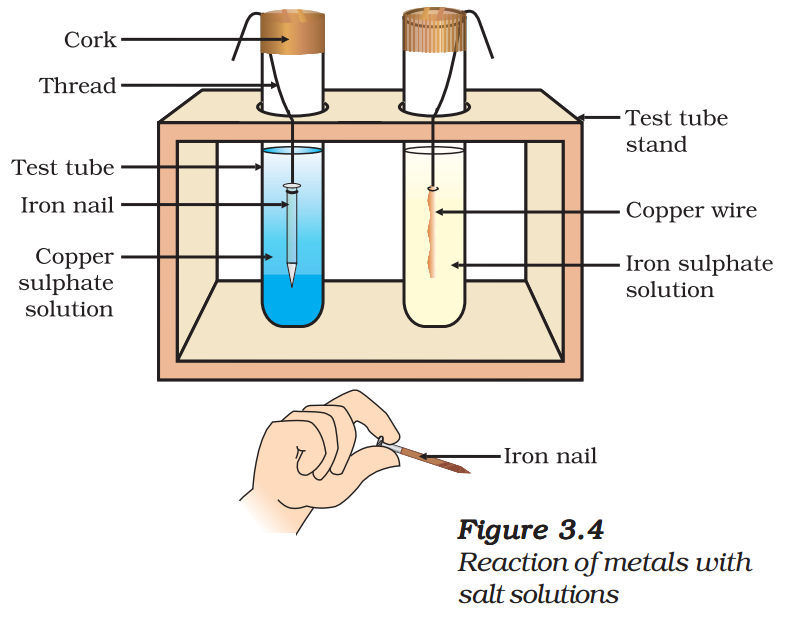
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
We have seen in the previous sections that all metals are not equally reactive. We checked the reactivity of various metals with oxygen, water and acids. But all metals do not react with these reagents. So we were not able to put all the metal samples we had collected in decreasing order of their reactivity. Displacement reactions studied in Chapter 1 give better evidence about the reactivity of metals. It is simple and easy if metal A displaces metal B from its solution, it is more reactive than B.
Metal A + Salt solution of B → Salt solution of A + Metal B
Which metal, copper or iron, is more reactive according to your observations in Activity 3.12?
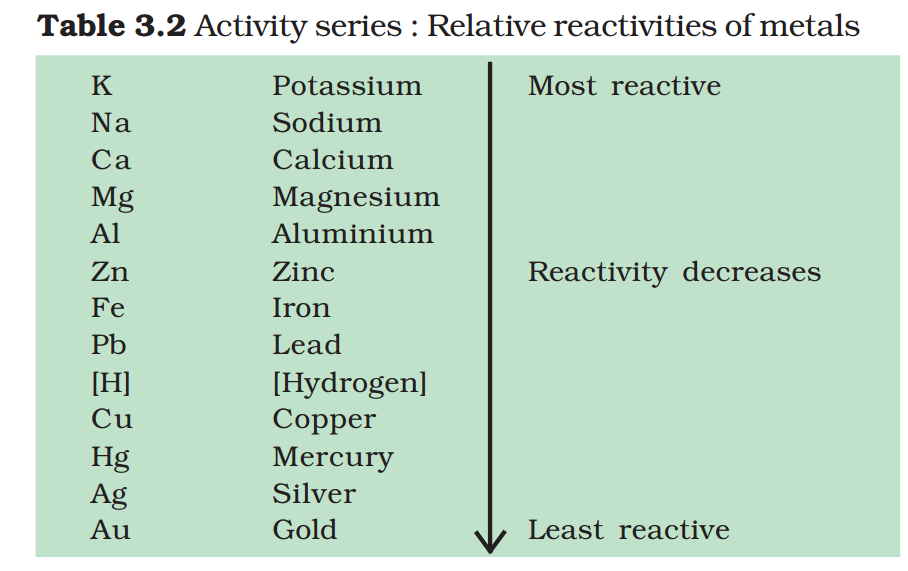
3.2.5 The Reactivity Series
The reactivity series is a list of metals arranged in the order of their decreasing activities. After performing displacement experiments (Activities 1.9 and 3.12), the following series, (Table 3.2) known as the reactivity or activity series has been developed.
Questions
1. Why is sodium kept immersed in kerosene oil?
2. Write equations for the reactions of
(i) iron with steam
(ii) calcium and potassium with water
3. Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
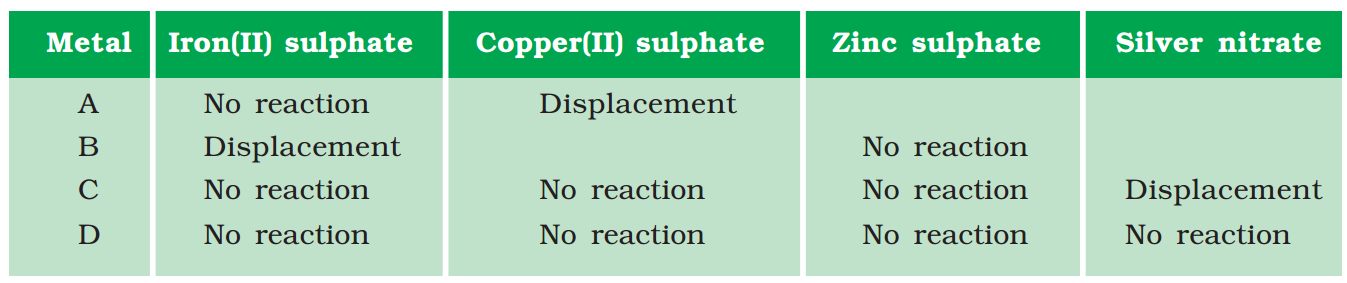
Use the Table above to answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal?
(ii) What would you observe if B is added to a solution of Copper(II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
4. Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H2SO4.
5. What would you observe when zinc is added to a solution of iron(II) sulphate? Write the chemical reaction that takes place.
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

