Trung Tâm Luyện Thi Đại Học
Chapter 2. Acids Bases and Salt
2.4. More About Salts
In the previous sections we have seen the formation of salts during various reactions. Let us understand more about their preparation, properties and uses.
Activity 2.13
- Write the chemical formulae of the salts given below. Potassium sulphate, sodium sulphate, calcium sulphate,
magnesium sulphate, copper sulphate, sodium chloride, sodium nitrate, sodium carbonate and ammonium chloride. - Identify the acids and bases from which the above salts may be obtained.
- Salts having the same positive or negative radicals are said to belong to a family. For example, NaCl and Na2SO4 belong to the family of sodium salts. Similarly, NaCl and KCl belong to the family of chloride salts. How many families can you identify among the salts given in this Activity?
2.4.2 pH of Salts
Activity 2.1.4
- Collect the following salt samples – sodium chloride, potassium nitrate, aluminium chloride, zinc sulphate, copper sulphate, sodium acetate, sodium carbonate and sodium hydrogencarbonate (some other salts available can also be taken).
- Check their solubility in water (use distilled water only).
- Check the action of these solutions on litmus and find the pH
using a pH paper. - Which of the salts are acidic, basic or neutral?
- Identify the acid or base used to form the salt.
- Report your observations in Table 2.4.

Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of a strong acid and weak base are acidic with pH value less than 7 and those of a strong base and weak acid are basic in nature, with pH value more than 7.
2.4.3 Chemicals from Common Salt
By now you have learnt that the salt formed by the combination of hydrochloric acid and sodium hydroxide solution is called sodium chloride. This is the salt that you use in food. You must have observed in the above Activity that it is a neutral salt.
Seawater contains many salts dissolved in it. Sodium chloride is separated from these salts. Deposits of solid salt are also found in several parts of the world. These large crystals are often brown due to impurities. This is called rock salt. Beds of rock salt were formed when seas of bygone ages dried up. Rock salt is mined like coal.
You must have heard about Mahatma Gandhi’s Dandi March. Did you know that sodium chloride was such an important symbol in our struggle for freedom?

Common salt — A raw material for chemicals
The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, bleaching powder and many more. Let us see how one substance is used for making all these different substances.
Sodium hydroxide
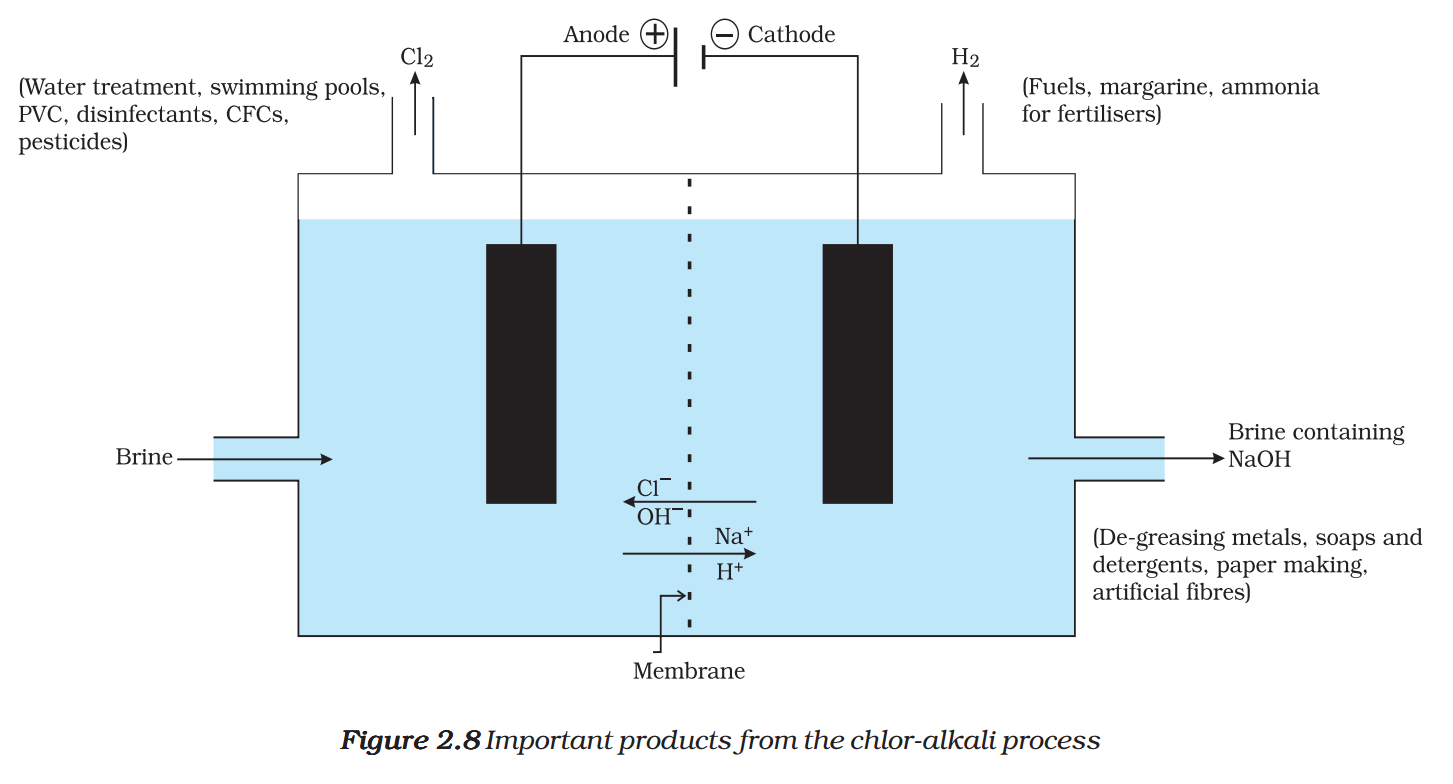
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
\( 2NaCl(aq)+2{{H}_{2}}O(\ell )\to 2NaOH(aq)+C{{l}_{2}}(g)+{{H}_{2}}(g) \)
Chlorine gas is given off at the anode, and hydrogen gas at the cathode. Sodium hydroxide solution is formed near the cathode. The three products produced in this process are all useful. Figure 2.8 shows the different uses of these products.
Bleaching powder
You have already come to know that chlorine is produced during the electrolysis of aqueous sodium chloride (brine). This chlorine gas is used for the manufacture of bleaching powder. Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2]. Bleaching powder is represented as CaOCl2, though the actual composition is quite complex.
\( Ca{{(OH)}_{2}}+C{{l}_{2}}\to CaOC{{l}_{2}}+{{H}_{2}}O \)
Bleaching powder is used –
(i) for bleaching cotton and linen in the textile industry, for bleaching wood pulp in paper factories and for bleaching washed clothes in laundry;
(ii) as an oxidising agent in many chemical industries; and
(iii) to make drinking water free from germs.
Baking soda
The baking soda is commonly used in the kitchen for making tasty crispy pakoras, etc. Sometimes it is added for faster cooking. The chemical name of the compound is sodium hydrogencarbonate (NaHCO3). It is produced using sodium chloride as one of the raw materials.
\( \begin{align} & NaCl+{{H}_{2}}O+C{{O}_{2}}+N{{H}_{3}}\to N{{H}_{4}}Cl\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,+\,\,\,\,\,\,\,\,\,\,\,\,\,\,NaHC{{O}_{3}} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(Ammonium\,\,chloride)\,\,\,\,\,(Sodium\,\,hydrogencarbonate) \\ \end{align} \)
Did you check the pH of sodium hydrogencarbonate in Activity 2.14?
Can you correlate why it can be used to neutralise an acid? It is a mild non-corrosive basic salt. The following reaction takes place when it is heated during cooking –
\( \begin{align} &2NaHC{{O}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\xrightarrow{Heat}\,\,\,\,N{{a}_{2}}C{{O}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,\,+\,\,\,\,\,\,\,\,\,\,\,\,\,{{H}_{2}}O+C{{O}_{2}} \\ & (Sodium\,\,hydrogencarbonate)\,\,\,\,\,(Sodium\,\,carbonate) \\ \end{align} \)
Sodium hydrogencarbonate has got various uses in the household.
Uses of Baking soda
(i) For making baking powder, which is a mixture of baking soda (sodium hydrogencarbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place –
\( \begin{align} & NaHC{{O}_{3}}+{{H}^{+}}\to C{{O}_{2}}+{{H}_{2}}O+Sodium\,\,salt\,\,of\,\,acid \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(From\,\,any\,\,acid) \\ \end{align} \)
Carbon dioxide produced during the reaction can cause bread or cake to rise making them soft and spongy.
(ii) Sodium hydrogencarbonate is also an ingredient in antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.
(iii) It is also used in soda-acid fire extinguishers.
Washing soda
Another chemical that can be obtained from sodium chloride is Na2CO3.H2O (washing soda). You have seen above that sodium carbonate can be obtained by heating baking soda; recrystallisation of sodium carbonate gives washing soda. It is also a basic salt.
\( \begin{align} & N{{a}_{2}}C{{O}_{3}}+10{{H}_{2}}O\to N{{a}_{2}}C{{O}_{3}}.{{H}_{2}}O \\ & (Sodium\,\,carbonate) \\ \end{align} \)
What does 10H2O signify? Does it make Na2CO3 wet? We will address this question in the next section.
Sodium carbonate and sodium hydrogencarbonate are useful chemicals for many industrial processes as well.
Uses of washing soda
(i) Sodium carbonate (washing soda) is used in glass, soap and paper industries.
(ii) It is used in the manufacture of sodium compounds such as borax.
(iii) Sodium carbonate can be used as a cleaning agent for domestic purposes.
(iv) It is used for removing permanent hardness of water.
2.4.4 Are the Crystals of Salts really Dry?
Activity 2.15
- Heat a few crystals of copper sulphate in a dry boiling tube.
- What is the colour of the copper sulphate after heating?
- Do you notice water droplets in the boiling tube? Where have these come from?
- Add 2-3 drops of water on the sample of copper sulphate obtained after heating.
- What do you observe? Is the blue colour of copper sulphate restored?
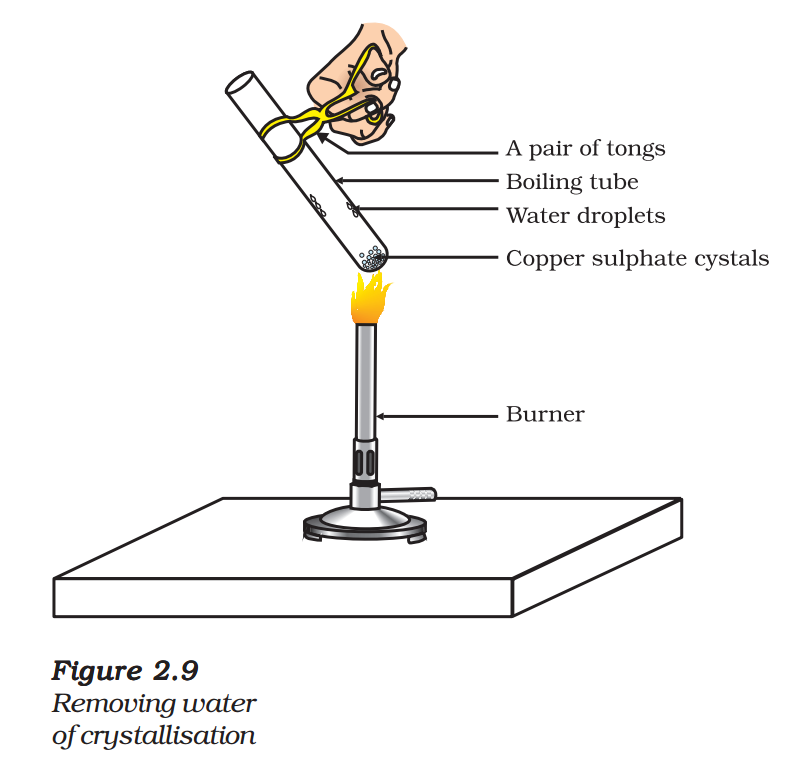
Copper sulphate crystals which seem to be dry contain water of crystallisation. When we heat the crystals, this water is removed and the salt turns white.
If you moisten the crystals again with water, you will find that blue colour of the crystals reappears.
Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formula unit of copper sulphate. Chemical formula for hydrated copper
sulphate is CuSO4. 5H2O. Now you would be able to answer the question whether the molecule of Na2CO3.10H2O is wet.
One other salt, which possesses water of crystallisation is gypsum.
It has two water molecules as water of cyrstallisation. It has the chemical formula CaSO4.2H2O. Let us look into the use of this salt.
Plaster of Paris
On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate \( \left( CaS{{O}_{4}}.\frac{1}{2}{{H}_{2}}O \right) \). This is called Plaster of Paris, the substance which doctors use as plaster for supporting fractured bones in the right position. Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass.
\( \begin{align} & CaS{{O}_{4}}.\frac{1}{2}{{H}_{2}}O+1\frac{1}{2}{{H}_{2}}O\to CaS{{O}_{4}}.2{{H}_{2}}O \\ & (Plaster\,\,of\,\,Paris)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(Gypsum) \\ \end{align} \)
Note that only half a water molecule is shown to be attached as water of crystallisation. How can you get half a water molecule? It is written in this form because two formula units of CaSO4 share one molecule of water. Plaster of Paris is used for making toys, materials for decoration and for making surfaces smooth. Try to find out why is calcium sulphate hemihydrate called ‘Plaster of Paris’ ?
Questions
- What is the common name of the compound CaOCl2?
- Name the substance which on treatment with chlorine yields bleaching powder.
- Name the sodium compound which is used for softening hard water.
- What will happen if a solution of sodium hydrocarbonate is heated?
Give the equation of the reaction involved. - Write an equation to show the reaction between Plaster of Paris and water.
What you have learnt
- Acid-base indicators are dyes or mixtures of dyes which are used to indicate the presence of acids and bases.
- Acidic nature of a substance is due to the formation of H+(aq) ions in solution.
Formation of OH–(aq) ions in solution is responsible for the basic nature of a substance. - When an acid reacts with a metal, hydrogen gas is evolved and a corresponding salt is formed.
- When a base reacts with a metal, along with the evolution of hydrogen gas a salt is formed which has a negative ion composed of the metal and oxygen.
- When an acid reacts with a metal carbonate or metal hydrogencarbonate, it gives the corresponding salt, carbon dioxide gas and water.
- Acidic and basic solutions in water conduct electricity because they produce hydrogen and hydroxide ions respectively.
- The strength of an acid or an alkali can be tested by using a scale called the pH scale (0-14) which gives the measure of hydrogen ion concentration in a solution.
- A neutral solution has a pH of exactly 7, while an acidic solution has a pH less than 7 and a basic solution a pH more than 7.
- Living beings carry out their metabolic activities within an optimal pH range.
- Mixing concentrated acids or bases with water is a highly exothermic process.
- Acids and bases neutralise each other to form corresponding salts and water.
- Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt.
- Salts have various uses in everyday life and in industries.
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

