Trung Tâm Luyện Thi Đại Học
Chapter 2. Acids Bases and Salt
2.3. How Strong Are Acid Or Base Solutions?
We know how acid-base indicators can be used to distinguish between an acid and a base. We have also learnt in the previous section about dilution and decrease in concentration of H+ or OH– ions in solutions.
Can we quantitatively find the amount of these ions present in a solution?
Can we judge how strong a given acid or base is?
We can do this by making use of a universal indicator, which is a mixture of several indicators. The universal indicator shows different colours at different concentrations of hydrogen ions in a solution.
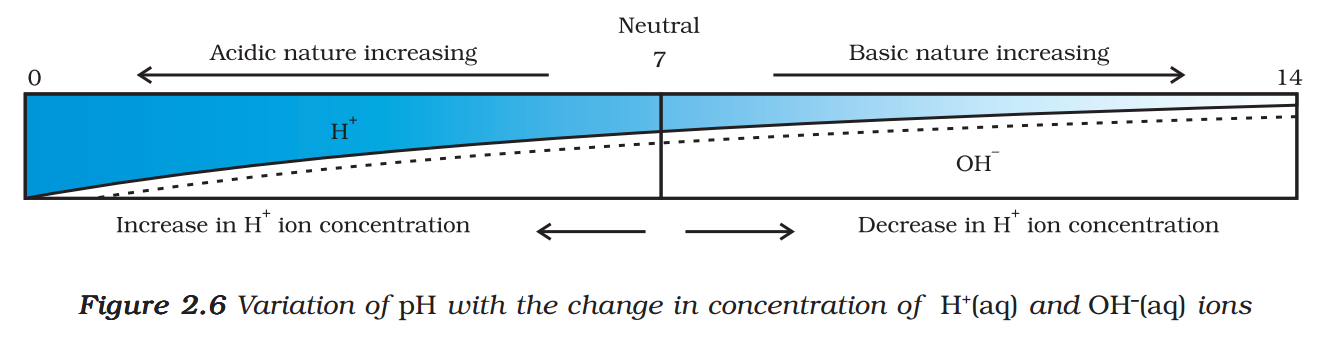
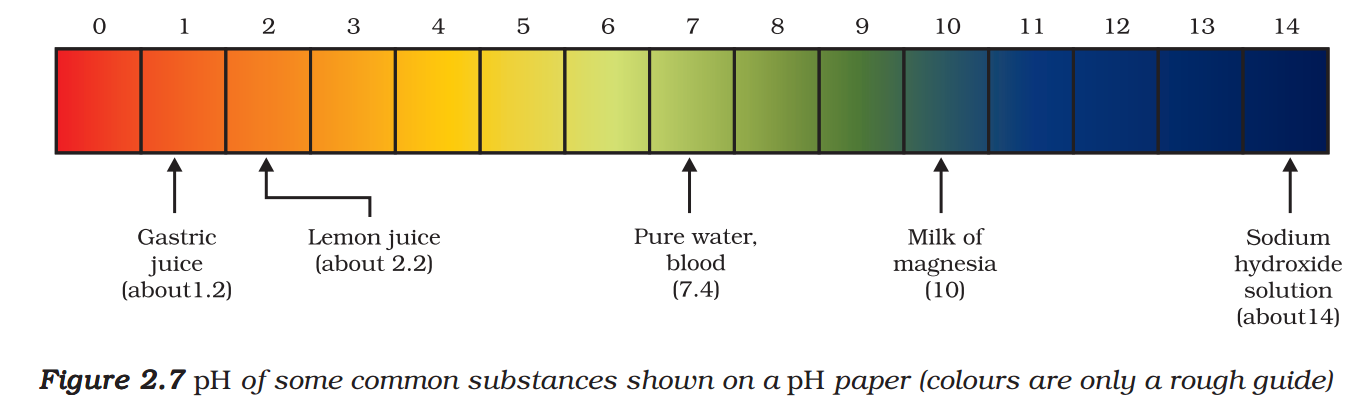
A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value.
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in OH– ion concentration in the solution, that is, increase in the strength of alkali (Fig. 2.6). Generally paper impregnated with the universal indicator is used for measuring pH.
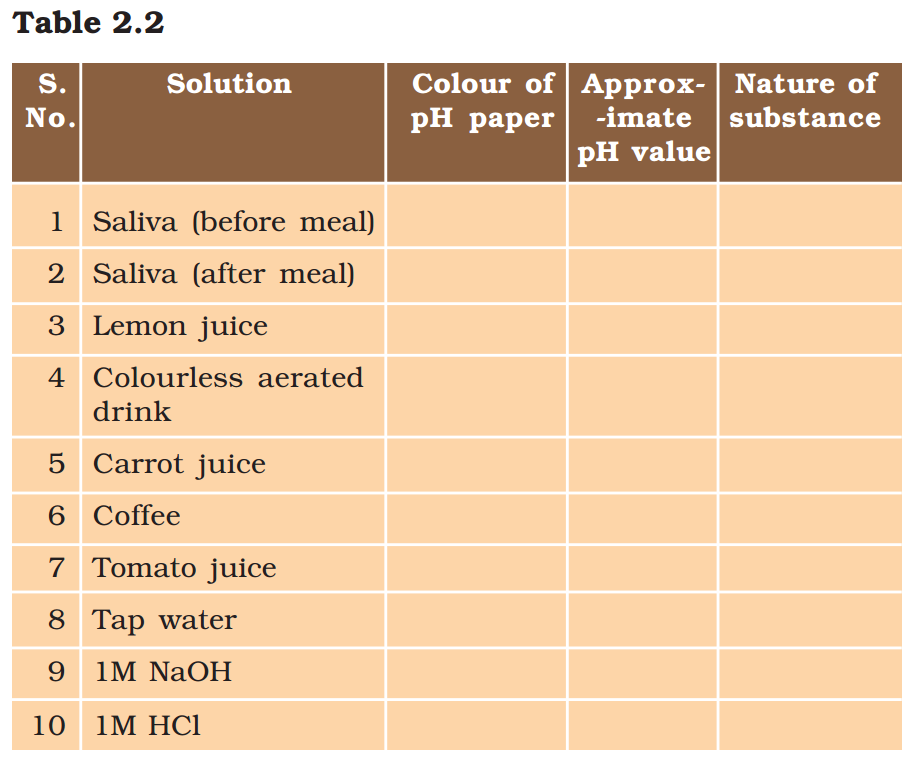
Activity 2.11
- Test the pH values of solutions given in Table 2.2.
- Record your observations.
- What is the nature of each substance on the basis of your observations?
The strength of acids and bases depends on the number of H+ ions and OH– ions produced, respectively. If we take hydrochloric acid and acetic acid of the same concentration, say one molar, then these produce different amounts of hydrogen ions. Acids that give rise to more H+ ions are said to be strong acids, and acids that give less H+ ions are said to be weak acids. Can you now say what weak and strong bases are?
2.3.1 Importance of pH in Everyday Life
Are plants and animals pH sensitive?
Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow range of pH change. When pH of rain water is less than 5.6, it is called acid rain. When acid rain flows into the rivers, it
lowers the pH of the river water. The survival of aquatic life in such rivers becomes difficult.
Do You Know?
Acids in other planets
The atmosphere of venus is made up of thick white and yellowish clouds of sulphuric acid. Do you think life can exist on this planet?
What is the pH of the soil in your backyard?
Plants require a specific pH range for their healthy growth. To find out the pH required for the healthy growth of a plant, you can collect the soil from various places and check the pH in the manner described below in Activity 2.12. Also, you can note down which plants are growing in the region from which you have collected the soil.
Activity 2.12
- Put about 2 g soil in a test tube and add 5 mL water to it.
- Shake the contents of the test tube.
- Filter the contents and collect the filtrate in a test tube.
- Check the pH of this filtrate with the help of universal indicator paper.
- What can you conclude about the ideal soil pH for the growth of plants in your region?
pH in our digestive system
It is very interesting to note that our stomach produces hydrochloric acid. It helps in the digestion of food without harming the stomach. During indigestion the stomach produces too much acid and this causes pain and irritation. To get rid of this pain, people use bases called antacids. One such remedy must have been suggested by you at the beginning of this Chapter. These antacids neutralise the excess acid. Magnesium hydroxide (Milk of magnesia), a mild base, is often used for this purpose.
pH change as the cause of tooth decay
Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made up of calcium hydroxyapatite (a crystalline form of calcium phosphate) is the hardest substance in the body. It does not dissolve in water, but is corroded when the pH in the mouth is below 5.5. Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. The best way to prevent this is to clean the mouth after eating food. Using toothpastes, which are generally basic, for cleaning the teeth can neutralise the excess acid and prevent tooth decay.
Self defence by animals and plants through chemical warfare
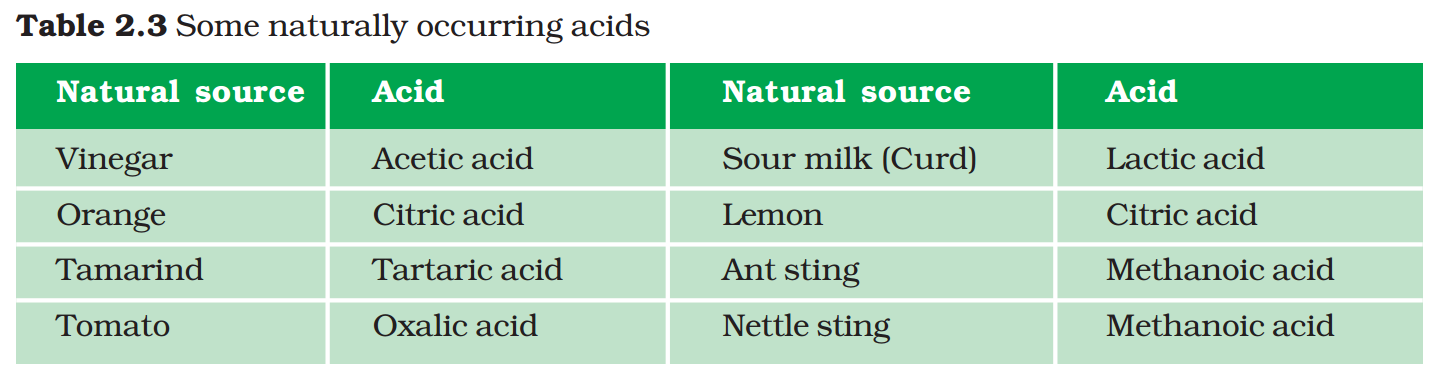
Have you ever been stung by a honey-bee? Bee-sting leaves an acid which causes pain and irritation. Use of a mild base like baking soda on the stung area gives relief. Stinging hair of nettle leaves inject methanoic acid causing burning pain.
Do You Know?

Nettle is a herbaceous plant which grows in the wild. Its leaves have stinging hair, which cause painful stings when touched accidentally. This is due to the methanoic acid secreted by them. A traditional remedy is rubbing the area with the leaf of the dock plant, which often grows beside the nettle in the wild. Can you guess the nature of the dock plant? So next time you know what to look out for if you accidentally touch a nettle plant while trekking. Are you aware of any other effective traditional remedies for such stings?
Questions
- You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic?
- What effect does the concentration of H+(aq) ions have on the nature of the solution?
- Do basic solutions also have H+(aq) ions? If yes, then why are these basic?
- Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

