Trung Tâm Luyện Thi Đại Học
Chapter 2. Acids Bases and Salt
You have lear tastes of food are due to acids and bases, respectively, present in them. nt in your previous classes that the sour and bitter
If someone in the family is suffering from a problem of acidity after overeating, which of the following would you suggest as a remedy– lemon juice, vinegar or baking soda solution?
- Which property did you think of while choosing the remedy? Surely you must have used your knowledge about the ability of acids and bases to nullify each other’s effect.
- Recall how we tested sour and bitter substances without tasting them.
You already know that acids are sour in taste and change the colour of blue litmus to red, whereas, bases are bitter and change the colour of the red litmus to blue. Litmus is a natural indicator, turmeric is another such indicator. Have you noticed that a stain of curry on a white cloth becomes reddish-brown when soap, which is basic in nature, is scrubbed on it? It turns yellow again when the cloth is washed with plenty of water. You can also use synthetic indicators such as methyl orange and phenolphthalein to test for acids and bases.
In this Chapter, we will study the reactions of acids and bases, how acids and bases cancel out each other’s effects and many more interesting things that we use and see in our day-to-day life.
Do You Know?
Litmus solution is a purple dye, which is extracted from lichen, a plant belonging to the division Thallophyta, and is commonly used as an indicator. When the litmus solution is neither acidic nor basic, its colour is purple. There are many other natural materials like red cabbage leaves, turmeric, coloured petals of some flowers such as Hydrangea, Petunia and Geranium, which indicate the presence of acid or base in a solution. These are called acid-base indicators or sometimes simply indicators.
Questions
- You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
2.1. Understanding The Chemical Properties Of Acids And Bases
2.1.1. Acids and Bases in the Laboratory
Activity 2.1
- Collect the following solutions from the science laboratory – hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2], potassium hydroxide (KOH), magnesium hydroxide [Mg(OH)2], and ammonium hydroxide (NH4OH).
- Put a drop of each of the above solutions on a watch-glass one by one and test with a drop of the indicators shown in Table 2.1.
- What change in colour did you observe with red litmus, blue litmus, phenolphthalein and methyl orange solutions for each of the solutions taken?
- Tabulate your observations in Table 2.1.
These indicators tell us whether a substance is acidic or basic by change in colour. There are some substances whose odour changes in acidic or basic media. These are called olfactory indicators. Let us try out some of these indicators.
Activity 2.2
- Take some finely chopped onions in a plastic bag along with some strips of clean cloth. Tie up the bag tightly and leave overnight in the fridge. The cloth strips can now be used to test for acids and bases.
- Take two of these cloth strips and check their odour.
- Keep them on a clean surface and put a few drops of dilute HCl solution on one strip and a few drops of dilute NaOH solution on the other.
- Rinse both cloth strips with water and again check their odour.
- Note your observations.
- Now take some dilute vanilla essence and clove oil and check their odour.
- Take some dilute HCl solution in one test tube and dilute NaOH solution in another. Add a few drops of dilute vanilla essence to both test tubes and shake well. Check the odour once again and record changes in odour, if any.
- Similarly, test the change in the odour of clove oil with dilute HCl and dilute NaOH solutions and record your observations.
Which of these – vanilla, onion and clove, can be used as olfactory indicators on the basis of your observations?
Let us do some more activities to understand the chemical properties of acids and bases.
2.1.2 How do Acids and Bases React with Metals?
Activity 2.3
CAUTION: This activity needs the teacher’s assistance.
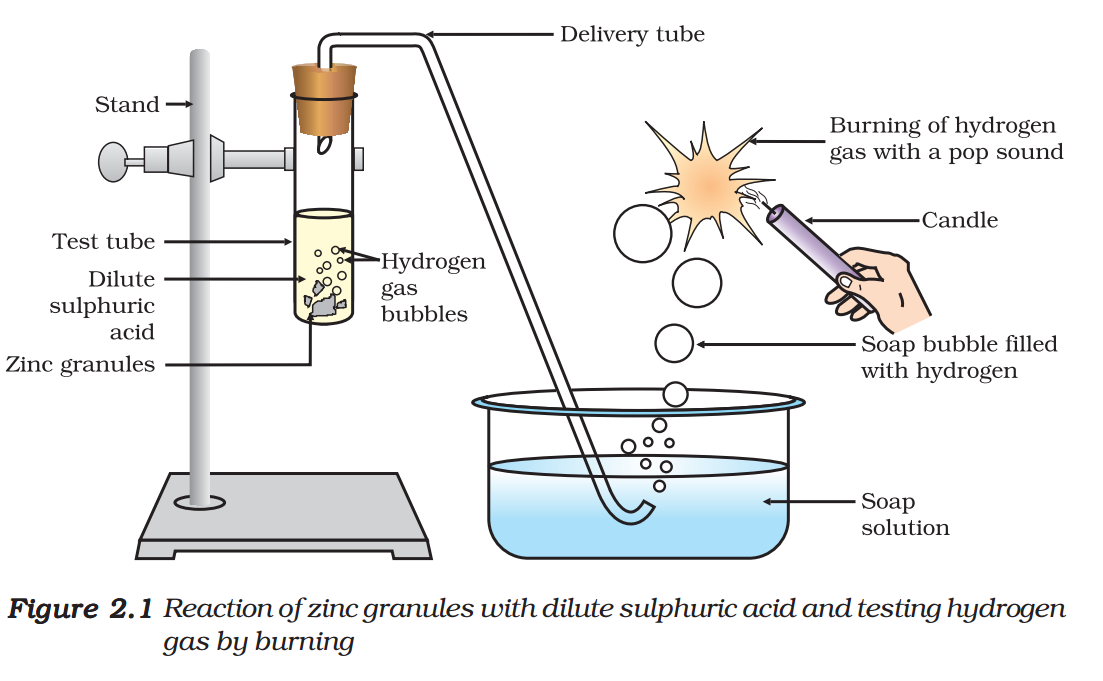
- Set the apparatus as shown in Fig. 2.1.
- Take about 5 mL of dilute sulphuric acid in a test tube and add a few pieces of zinc granules to it.
- What do you observe on the surface of zinc granules?
- Pass the gas being evolved through the soap solution.
- Why are bubbles formed in the soap solution?
- Take a burning candle near a gas filled bubble.
- What do you observe?
- Repeat this Activity with some more acids like HCl, HNO3 and CH3
- Are the observations in all the cases the same or different?
Note that the metal in the above reactions displaces hydrogen atoms from the acids as hydrogen gas and forms a compound called a salt.
Thus, the reaction of a metal with an acid can be summarised as –
Acid + Metal → Salt + Hydrogen gas
Can you now write the equations for the reactions you have observed?
Activity 2.4
- Place a few pieces of granulated zinc metal in a test tube.
- Add 2 mL of sodium hydroxide solution and warm the contents of the test tube.
- Repeat the rest of the steps as in Activity 2.3 and record your observations.
The reaction that takes place can be written as follows.
\( \begin{align} & 2NaOH(aq)+Zn(s)\to N{{a}_{2}}Zn{{O}_{2}}(s)+{{H}_{2}}(g) \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(Sodium\,\,zincate) \\ \end{align} \)
You find again that hydrogen is formed in the reaction. However, such reactions are not possible with all metals.
1.2.3 How do Metal Carbonates and Metal Hydrogencarbonates React with Acids?
Activity 2.5
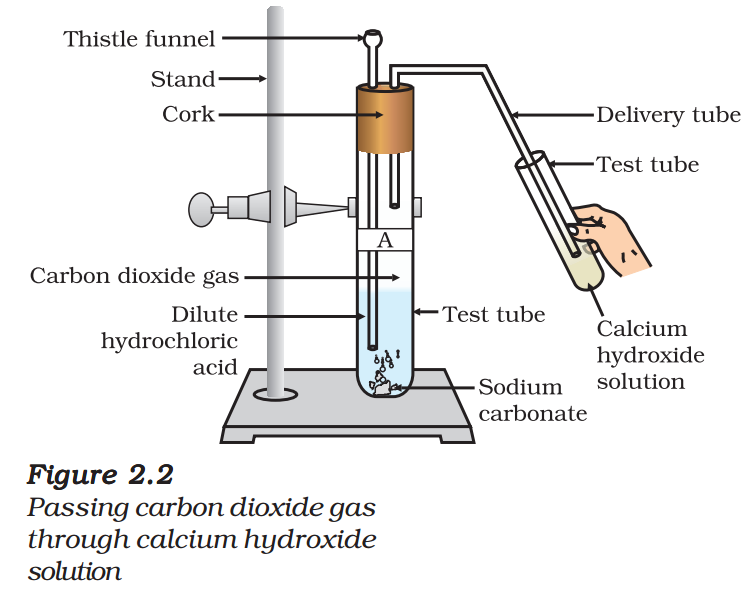
- Take two test tubes, label them as A and B.
- Take about 0.5 g of sodium carbonate (Na2CO3) in test tube A and about 0.5 g of sodium hydrogencarbonate
(NaHCO3) in test tube B. - Add about 2 mL of dilute HCl to both the test tubes.
- What do you observe?
- Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in Fig. 2.2 and record your observations.
The reactions occurring in the above Activity are written as –
Test tube A: \( N{{a}_{2}}C{{O}_{3}}(s)+2HCl(aq)\to 2NaCl(aq)+{{H}_{2}}O(\ell )+C{{O}_{2}}(g) \)
Test tube B: \( NaHC{{O}_{3}}(s)+HCl(aq)\to NaCl(aq)+{{H}_{2}}O(\ell )+C{{O}_{2}}(g) \)
On passing the carbon dioxide gas evolved through lime water,
\( \begin{align} & Ca{{(OH)}_{2}}(aq)+C{{O}_{2}}(g)\to CaC{{O}_{3}}(s)+{{H}_{2}}O(\ell ) \\ & (Lime\,\,water)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(White\,\,precipitate) \\ \end{align} \)
On passing excess carbon dioxide the following reaction takes place:
\( \begin{align} & CaC{{O}_{3}}(s)+{{H}_{2}}O(\ell )+C{{O}_{2}}(g)\to Ca(HC{{O}_{3}})(aq) \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{Soluble}\,\,\text{in}\,\,\text{water}) \\ \end{align} \)
Limestone, chalk and marble are different forms of calcium carbonate. All metal carbonates and hydrogencarbonates react with acids to give a corresponding salt, carbon dioxide and water.
Thus, the reaction can be summarised as –
Metal carbonate/Metal hydrogencarbonate + Acid → Salt + Carbon dioxide + Water
2.1.4 How do Acids and Bases React with each other?
Activity 2.6
- Take about 2 mL of dilute NaOH solution in a test tube and add two drops of phenolphthalein solution.
- What is the colour of the solution?
- Add dilute HCl solution to the above solution drop by drop.
- Is there any colour change for the reaction mixture?
- Why did the colour of phenolphthalein change after the addition of an acid?
- Now add a few drops of NaOH to the above mixture.
- Does the pink colour of phenolphthalein reappear?
- Why do you think this has happened?
In the above Activity, we have observed that the effect of a base is nullified by an acid and vice-versa. The reaction taking place is written as –
\( NaOH(aq)+HCl(aq)\to NaCl(aq)+{{H}_{2}}O(\ell ) \)
The reaction between an acid and a base to give a salt and water is known as a neutralisation reaction. In general, a neutralisation reaction can be written as –
Base + Acid → Salt + Water
2.1.5 Reaction of Metallic Oxides with Acids
Activity 2.7
- Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly while stirring.
- Note the colour of the solution. What has happened to the copper oxide?
You will notice that the colour of the solution becomes blue-green and the copper oxide dissolves. The blue-green colour of the solution is due to the formation of copper(II) chloride in the reaction. The general reaction between a metal oxide and an acid can be written as –
Metal oxide + Acid → Salt + Water
Now write and balance the equation for the above reaction. Since metallic oxides react with acids to give salts and water, similar to the reaction of a base with an acid, metallic oxides are said to be basic oxides.
2.1.6 Reaction of a Non-metallic Oxide with Base
You saw the reaction between carbon dioxide and calcium hydroxide (lime water) in Activity 2.5. Calcium hydroxide, which is a base, reacts with carbon dioxide to produce a salt and water. Since this is similar to the reaction between a base and an acid, we can conclude that nonmetallic oxides are acidic in nature.
Questions
1. Why should curd and sour substances not be kept in brass and copper vessels?
2. Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
3. Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds
formed is calcium chloride.
Các bài toán cùng chủ đề!
Các sách luyện thi do Trung tâm phát hành!
Trung Tâm Luyện Thi Đại Học được xây dựng trên WordPress

